Fig. 5. Neotelomere formation at Cas9 enzyme-product complexes.
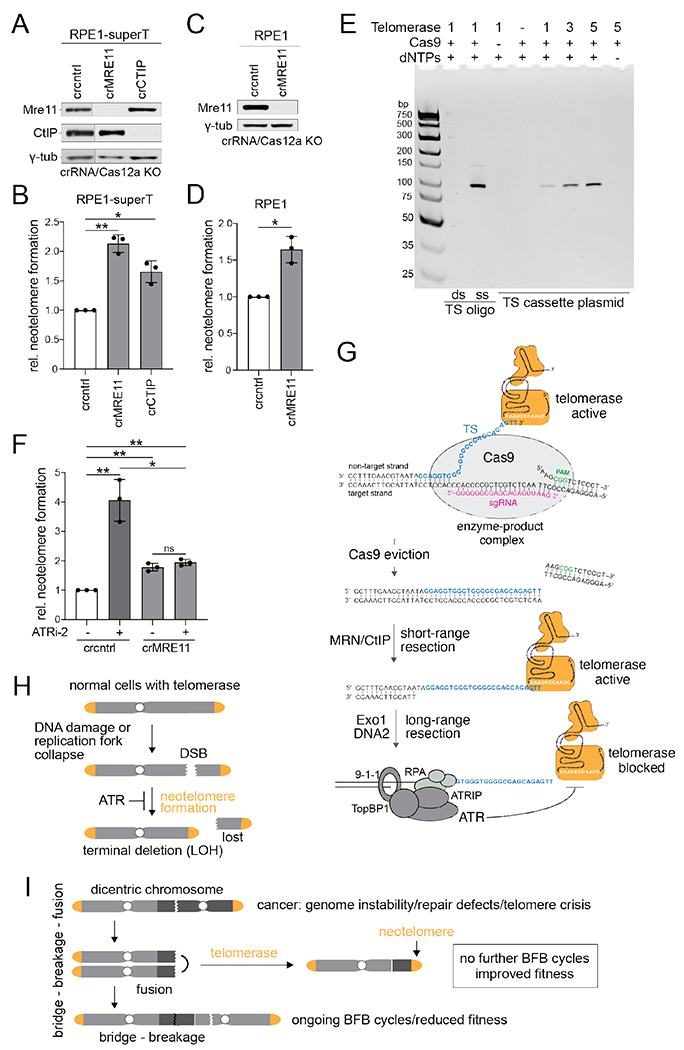
(A) Immunoblot for the indicated proteins from RPE1-superT cells treated with Cas12a and crRNAs targeting MRE11 or CtIP or a control crRNA. γ-tub, loading control. The blot was cut between lanes 1 and 2 to remove lanes corresponding to irrelevant samples. (B) Relative neotelomere formation based on qPCR in cells in (A). (C) Immunoblot for the indicated proteins 5 in pLenti-sgTS-TaqMan-TS RPE1 cells treated with Cas12a and crRNAs targeting MRE11 or a control (cntrl) crRNA. γ-tub, loading control. (D) Relative neotelomere formation based on qPCR in cells in (C). (E) In vitro assay for telomerase acting at the Cas9 enzyme-product complex. In vitro telomeric repeat addition by telomerase at the TS cut site after incubating the TS cassette plasmid with Cas9 and sgTS (Fig. 1A) was detected by EthBr staining of the generated PCR products, as in Fig. 1B. Control oligonucleotides represent the Cas9 cut at TS in ss and ds form. (F) Relative neotelomere formation based on qPCR in pLenti-sgTS-TaqMan-TS RPE1 cells targeted for MRE11 with or without ATRi. Mean ± SD of 3 biological replicates. ns p > 0.05, * p < 0.05, ** p < 0.01, two-tailed ratio-paired t-test. ATRi-2, gartisertib/M4344, 0.3 μM. (G) Model for neotelomere formation at Cas9-induced DSBs in the presence and absence of MRN/CtIP, highlighting two priming sites for telomerase. When Cas9 persists, the extruded non-target strand is the substrate, and MRN/CtIP is not required. When Cas9 is evicted, short-range resection by MRN/CtIP is required to generate the free 3’ end for telomerase priming. Long-range resection activates ATR, which inhibits telomerase at the resected DSB. (H) Neotelomere formation in normal human cells is limited by the low (or no) telomerase expression and/or by ATR signaling at resected DSBs or regressed replication forks. Neotelomere formation induces terminal deletions and loss of heterozygosity (LOH) for distal genes. (I) Potential role for neotelomere formation in cancer. Neotelomere formation may enhance cancer genome evolution by enforcing LOH (as in (H)) and end ongoing BFB cycles in genomically unstable cancer clones, thereby enhancing their fitness.
