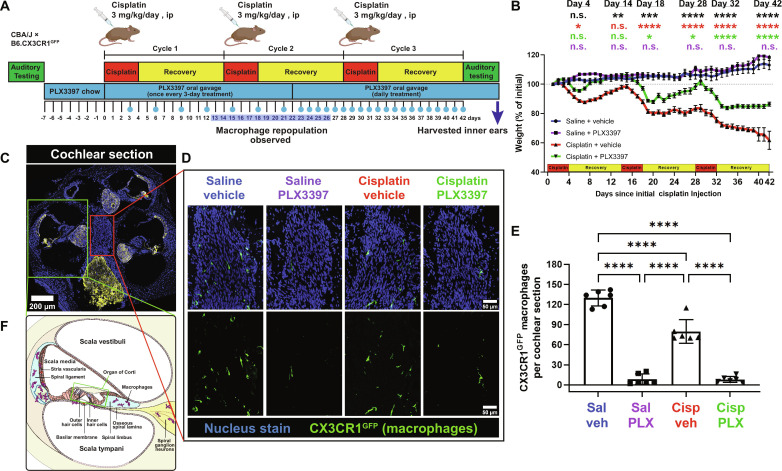
Fig. 1. Initial protocol in experiment 1 resulted in macrophage ablation via PLX3397, followed by partial macrophage repopulation.
(A) Experimental design showing auditory tests, PLX3397 treatment, and 3 cycles of cisplatin administration. Mice received PLX3397-formulated chow for 7 days to facilitate macrophage depletion. During cisplatin administration, mice received PLX3397 via oral gavage once every 3 days. Upon observation of macrophage repopulation on day 14 (see fig. S1), daily treatment of PLX3397 via oral gavage was initiated. The days on which mice received PLX3397 via oral gavage are denoted with blue circles. ip, intraperitonally. (B) Changes in mouse weight are shown throughout the 3 cycles of cisplatin administration protocol. Means ± SEM, n = 10 to 16 mice per experimental group (total 49 mice; 24 females and 25 males). Statistical comparisons (asterisks or n.s.) are color-coded as described in Methods. (C) Cochleae harvested after end-point auditory tests were immunolabeled for Kir4.1 (yellow) to visualize cochlear structures and green fluorescent protein (GFP) to visualize CX3CR1GFP-positive macrophages (green). Statistical significance is indicated as the following: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. P value above 0.05 (P > 0.05) was considered nonsignificant (n.s.). (D) Representative confocal images [cochlear modiolus; from red box in (C)] and (E) quantification of macrophages per cochlear section. PLX3397 resulted in >93% depletion of all macrophages. (F) Schematic diagram of macrophage distribution in the middle cochlear turn [from green box in (C)]. Scale bars, 200 μm (cochlear section) and 50 μm (mid-modiolar section). Means ± SD, n = 6 cochleae (3 females and 3 males) per experimental group (total 24 cochleae; 12 females and 12 males). One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test.