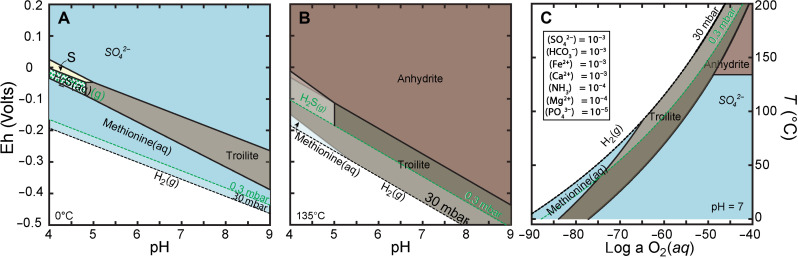
Fig. 4. Eh-pH (Pourbaix) diagram for stable sulfur compounds in Ryugu.
Sulfur species stability boundaries (Eh-pH diagrams) calculated as a function of redox potential and pH at 0°C (273 K) (A) and 135°C (408 K) (B), and S species and as a function of temperature from 0° to 200°C (273 to 473 K) and log O2 activity from −90 to −40 M at pH = 7 (C). Solid black lines define sulfur phase and species boundaries at 30 mbar, and green lines show the same boundaries at 0.3 mbar. Blue fields are aqueous; the stability field of troilite (Fe1−xS) is tan, that of anhydrite (CaSO4) is red, and that of gaseous hydrogen sulfide (H2S) is white. Aqueous H2S coexists with gaseous H2S in regions marked with green hashed lines. Black dashed lines show the stability boundary of water and H2(g) at 30 mbar, and stippled green lines show the H2(g)- H2O boundary shifts from 30 to 0.3 mbar. Model input solution composition data [shown as inset in (C)] are assumed from (3) and constrained by known C-type asteroids; temperature limits are assumed to be < ~150°C for Ryugu particles (6, 38). Activities of species yet unreported were assigned a log activity of 10−3, an assumption consistent with known constraints from C-type asteroids. Pyrite precipitation was suppressed in the model. The broad predominance field of stable methionine across the wide pH range at all low-O2 conditions demonstrates thiol species in close association with sulfate and sulfides are thermodynamically permissible.