Abstract
Background:
Interleukin 13 (IL-13) is a pleiotropic cytokine that has been shown to be important in the pathogenesis of chronic rhinosinusitis with nasal polyps (CRSwNP) and other type 2 inflammation–related diseases. Increased IL-13 expression can elicit several pro-inflammatory effects, including eosinophilia, and pathology such as increased mucus secretion. Polypogenesis in chronic rhinosinusitis (CRS) can be caused by hypoxia, which can also lead to hyperpermeability of airway epithelium and epithelium-to-mesenchymal translation through the upregulation of hypoxia-associated genes, such as HIF1. Whether T-helper 2 (Th2) inflammatory cytokines, such as IL-13, can also induce sinonasal epithelial hypoxia-associated genes is currently unknown.
Methods:
Human air-liquid interface (ALI) sinonasal epithelial cell cultures treated with recombinant IL-13 were analyzed by real-time polymerase chain reaction (PCR) and flow cytometry to determine the effect on epithelial cells.
Results:
Whole tissue from CRSwNP subjects showed increased HIF1A gene expression. Treatment of fully differentiated human ALI cultures with IL-13 resulted in a concurrent increase in HIF1A and ARNT messenger RNA (mRNA) expression. However, the level of EPAS1 expression was significantly reduced. IL-13 also had a dose-dependent response on the expression of HIF genes and the time course experiment showed peak expression of HIF1A and ARNT at 5 to 7 days poststimulation. Remarkably, CD73 surface expression also peaked at day 5 poststimulation.
Conclusion:
Our data suggests that IL-13 can induce hypoxia signaling pathway genes leading to surface expression of CD73, which has an anti-inflammatory effect.
Keywords: sinonasal epithelium, hypoxia, IL-13, cell culture, CD73
Chronic rhinosinusitis (CRS) includes a broad spectrum of conditions characterized by persistent inflammation of the sinonasal mucosa for at least 12 weeks.1 The disorder is estimated to affect up to 12% of population in the United States and incurs significant economic cost.2 CRS with nasal polyps (CRSwNP) is typically characterized by a greater burden of disease with increased inflammation and epithelial permeability, infiltration of immune cells, and mucosal remodeling. CRSwNP is associated with increased expression of type 2 cytokines by epithelial cells (interleukin [IL]-25, IL-33, and thymic stromal lymphopoietin [TSLP]) and T-helper 2 (Th2) immune cells (IL-5, IL-13). IL-13 is associated with the development of polyps and can initiate recruitment of lymphocytes, eosinophils, basophils, mast cells, and differentiation of monocytes into macrophages.3–5 The importance of IL-13 signaling is suggested by the often dramatic clinical response to dupilumab in CRSwNP patients, although the precise mechanism through which the IL-4/IL-13 receptor antibody reverses the disease process is not fully understood.3
Reduced oxygen tension, or hypoxia, is a major pathophysiological sign in sinusitis, and the disease severity is correlated with the oxygen level.6 In general, hypoxia leads to the activation of a signaling pathway through translocation of hypoxia-induced factor-α protein subunits (HIF-1α [HIF1A], HIF-2α [EPAS1]) to the nucleus and binding with HIF-β (HIF-1β [ARNT]). A variety of downstream effector functions of the hypoxia signaling pathway have been described, including cellular metabolic adaptation through anaerobic glycolysis, expression of proteins such as vascular endothelial growth factor (VEGF) and erythropoietin, and regulation of inflammatory myeloid cells. Additionally, the hypoxia signaling pathway can lead to anti-inflammatory responses, such as HIF-1α binding to the promoter region of CD73, which leads to an increase in ecto-5′-nucleotidase (CD73) enzyme level.7 CD73 is a membrane-bound glycoprotein that assists in conversion of “danger signals” adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP) into anti-inflammatory adenosine.
In the present study, we show that IL-13 is sufficient to induce the hypoxia signaling pathway. We also show that the expression of hypoxia signaling pathway genes is IL-13 dose-dependent and peaks at 5 to 7 days poststimulation. The peak expression of hypoxia signaling pathway proteins corresponds with the surface expression of anti-inflammatory CD73 proteins. Together our data suggest (1) a novel mode of induction of hypoxia through IL-13, and (2) a possible anti-inflammatory effect of IL-13 in sinonasal epithelial cells.
Patients and methods
Human subjects
Sinonasal tissue from 22 CRSwNP and 14 control subjects were utilized in this study. The Johns Hopkins Institutional Review process approved the research protocol and all subjects signed informed consent statement. The CRSwNP patients were characterized by continuous symptoms of rhinosinusitis for at least 12 weeks as defined by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) Chronic Rhinosinusitis Task Force and the EPOS guidelines.1–8 Computed tomography (CT) of the sinuses revealed isolated or diffuse sinus mucosal thickening or air-fluid levels, and nasal polyps visible on diagnostic endoscopy. Participating subjects had no history of tobacco use, cystic fibrosis, ciliary dyskinesia, systemic inflammatory or autoimmune disease, or immunodeficiency. Control subjects are defined as those without any sign of CRS undergoing endoscopic sinonasal procedure for cerebrospinal fluid leak repair, orbital decompression, or benign sinus lesion removal. All tissue samples were obtained from either resected uncinate process or anterior ethmoid sinus.
Sinus tissue processing
Human sinus tissue was digested with 0.05 mg/mL Liberase TL (Roche Diagnostics, Mannheim, Germany) and 0.5 mg/mL DNaseI (Sigma-Aldrich, St. Louis, MO) for 45 minutes at 37°C and 5% CO2. The digested tissue was filtered through a 70-μm cell strainer (BD Biosciences, San Jose, CA, USA). Liberase was inactivated with the addition of Roswell Park Memorial Institute (RPMI) 1640 culture medium (Corning, Corning, NY) media with 10% fetal bovine serum (FBS; Sigma-Aldrich). After centrifugation, the pellet was treated with ammonium-chloride-potassium (ACK) Lysis Buffer to lyse red blood cells. The lysis was stopped with RPMI (10% FBS) media and the cells were refiltered with a 40-μm cell strainer (BD Biosciences) followed by centrifugation. The pellet was resuspended in media and counted by trypan blue exclusion method. Epithelial cells were separated from tissue using enzymatic digestion and grown in human collagen (Type IV; Sigma-Aldrich)-coated culture dishes and expanded to confluence in expansion media (Pneumacult-Ex Plus; Stemcell Technologies, Vancouver, Canada). Upon confluency, the submerged cells were washed with phosphate-buffered saline (PBS) followed by trypsinization for 5 to 10 minutes.
Air-liquid interface culture
Epithelial cells were seeded onto collagen-IV–coated (Sigma-Aldrich) transwell plates (Corning) and expanded to confluence in expansion media (Pneumacult-Ex Plus). After confluent, the apical media was aspirated and the basolateral media was replaced with air-liquid interface (ALI) media (Pneumacult-ALI; Stemcell Technologies). The cells are considered fully differentiated after 21 days of ALI. After at least 3 weeks of differentiation, the ALI cells are treated with 50 ng/mL recombinant human IL-13 (BioLegend, San Diego, CA). At each experimental time point, the ALI cells were washed with PBS and treated with Accutase (Stemcell Technologies) for 30 minutes at 37°C. The detached cells strained through a 70-μm cell strainer before being plated in a 96-well round bottom tissue culture plate for flow cytometry staining.
RNA extraction and real-time polymerase chain reaction
RNA was extracted from frozen human sinonasal tissue or ALI cultured cells using TRIzol (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was generated by reverse transcribing 0.15 to 1 μg of RNA using SuperScript III and random primers (Invitrogen kit). cDNA was diluted 5-fold to 10-fold using double-distilled water before polymerase chain reaction (PCR). Real-time PCR was performed using appropriate TaqMan Fast Advanced master mix and TaqMan primer probes (Thermo Fisher Scientific, Waltham, MA): HIF1A (Hs00153153_m1), ARNT (Hs01121918_m1), EPAS1 (Hs01026149_m1), ARNT2 (Hs00977663_m1), and HIF3A (Hs00541709_m1).
Flow cytometry
Detached culture cells were washed with PBS and labeled with a live/dead dye (Zombie Aqua; BioLegend) for 15 minutes at room temperature (RT) and in the dark. After centrifugation, the cells were blocked with Fc receptor binding inhibitor monoclonal antibody (Anti-human; eBioscience, Santa Clara, CA) in Flow Buffer (PBS + 2% FBS + 2mM ethylenediamine tetraacetic acid [EDTA]) for 30 minutes at 4°C. For the detection of epithelial cell population, cells were stained with Ax700-conjugated anti-CD45 (BioLegend), Ax700-conjugated anti-CD31 (BioLegend), Ax700-conjugated anti-CD90 (BioLegend), and fluorescein isothiocyanate (FITC)-conjugated anti-CD73 (BioLegend). Samples and controls were analyzed on an LSRII flow cytometer (BD Biosciences) using FACSDiVa (BD Biosciences). Acquired data were gated to include only single cells (forward light scatter width [FSC-W]/forward light scatter area [FSC-A] and side scatter width [SSC-W]/side scatter area [SSC-A]) that were live (Aqua−). Positive cellular expression of markers was gated based on fluorescence minus one (FMO) controls.
Statistical analysis
Statistical differences were determined using Mann-Whitney t test or 1-way analysis of variance (ANOVA) in GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Results
Hypoxia-related genes in human sinonasal epithelium
Hypoxia can develop in chronic airway diseases such as sinusitis and asthma due to alterations in microvascular structures and increased metabolism.9 Published literature demonstrates local activation of the hypoxia signaling pathway with effects such as mucus hypersecretion,10 increased vascular permeability through VEGF expression,11 and reduced expression of junction proteins.12 Other studies have demonstrated an impact of hypoxia on immune cells.13 However, sinonasal epithelial expression of hypoxia-related genes in CRSwNP has not been comprehensively evaluated relative to healthy controls. To assess overall expression, we performed quantitative PCR analysis of HIF1A, ARNT, EPAS1, ARNT2, and HIF3A expression in whole tissue samples (Fig. 1). HIF1A expression was significantly higher in CRSwNP tissue compared to healthy sinonasal mucosa, while the expression of a constitutively expressed nuclear subunit (ARNT) was not different. On the other hand, EPAS1 expression was significantly lower in CRSwNP samples. ARNT2 and HIF3A expression was similar between CRSwNP and control subjects.
FIGURE 1.
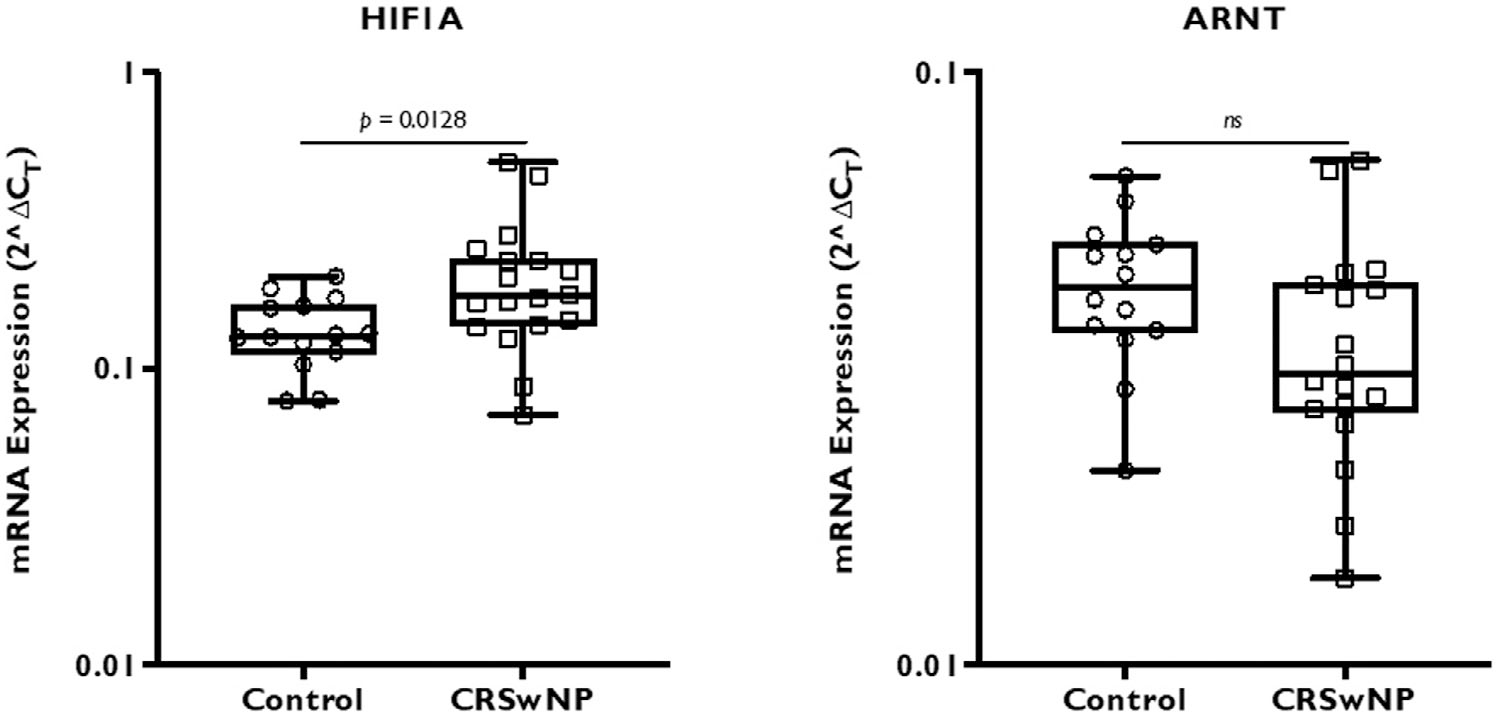
Whole tissue HIF gene expression of CRSwNP and control subjects. mRNA expression of HIF genes from a total of 14 control and 18 CRSwNP subjects were analyzed by real-time PCR. The results are represented as 2ΔCT, where ΔCT was calculated by normalizing target gene CT value to β-actin housekeeping gene. Statistics was calculated using Mann-Whitney nonparametric t test with significance at p < 0.05. CRSwNP = chronic rhinosinusitis with nasal polyps; CT = threshold cycle; HIF = hypoxia-inducible factor; mRNA = messenger RNA; PCR = polymerase chain reaction.
IL-13 can affect hypoxia-signaling pathway genes
Although oxygen tension is considered to be the main regulator of HIF activity, non-hypoxic activation of HIF through inflammatory cytokines IL-1, IL-6, and tumor necrosis factor (TNF) has been described.14 Among the HIF proteins, HIF-1α (HIF1A) and HIF-2α (EPAS1) are closely related subunits that have similar properties.15 However, Crotty Alexander et al.16 showed that HIF-1α and HIF-2α regulate eosinophil migration in opposing ways, with deletion of HIF-1α in myeloid cells of a mouse asthma model reducing eosinophil infiltration.
To test whether hypoxia-related gene expression is altered by a well-known type 2 cytokine, we treated fully differentiated ALI culture cells for 2 weeks with 50 ng/mL of recombinant IL-13. The results in Figure 2 represent 4 independent experiments. HIF1A was significantly upregulated while ARNT showed a similar trend, although this upregulation was not statistically significant. Conversely, EPAS1 showed a trend toward downregulation. Elsewhere, we observed significant downregulation of EPAS1 gene expression in IL-13 treated cells. These experiments strongly suggest that IL-13 can activate HIF pathway genes, HIF1A and ARNT, independent of oxygen supply, while downregulating EPAS1 expression in the sinonasal epithelial cells.
FIGURE 2.
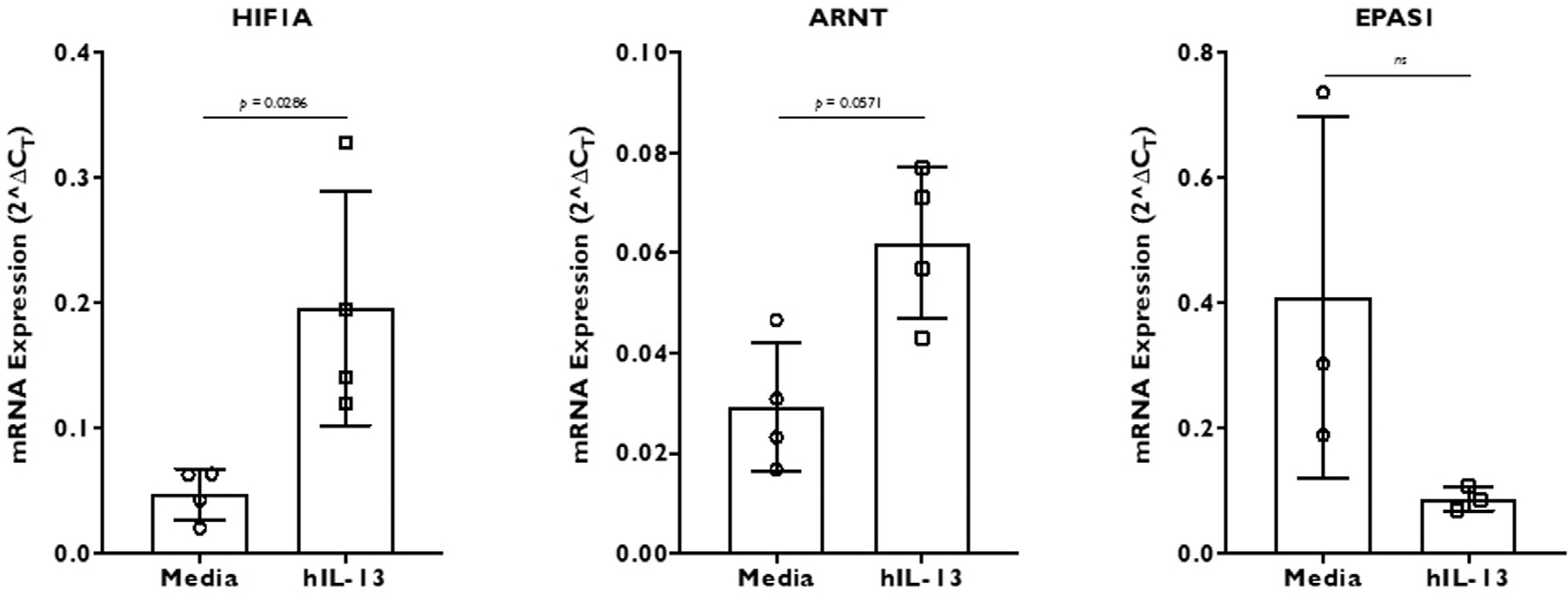
HIF gene (HIF1A, ARNT, and EPAS1) expression in recombinant human IL-13 stimulated differentiated ALI culture cells. The cells were stimulated for 2 weeks before RNA extraction and real-time PCR. The results represent 4 independent experiments with 2 to 4 replicates in each experiment (1 experiment with 2 replicates, 2 experiments with 3 replicates, and 1 experiment with 4 replicates of each condition). The results are represented as 2ΔCT, where ΔCT was calculated by normalizing target gene CT value to β-actin housekeeping gene. Data are represented as mean ± SD. Statistics was calculated using Mann-Whitney nonparametric t test with significance at p < 0.05. ALI = air-liquid interface; CT = threshold cycle; HIF = hypoxia-inducible factor; IL = interleukin; PCR = polymerase chain reaction; SD = standard deviation.
Effect of IL-13 on hypoxia-signaling pathway is dose-dependent and time-dependent
We next sought to determine whether the effect of IL-13 on sinonasal epithelial cells is dose-dependent and time-dependent. Although 1 ng/mL and 10 ng/mL IL-13 treatment over 2 weeks showed insignificant to modest effect, 50 ng/Ml caused significant changes in gene expression of HIF1A, ARNT, and EPAS1 (Fig. 3).
FIGURE 3.
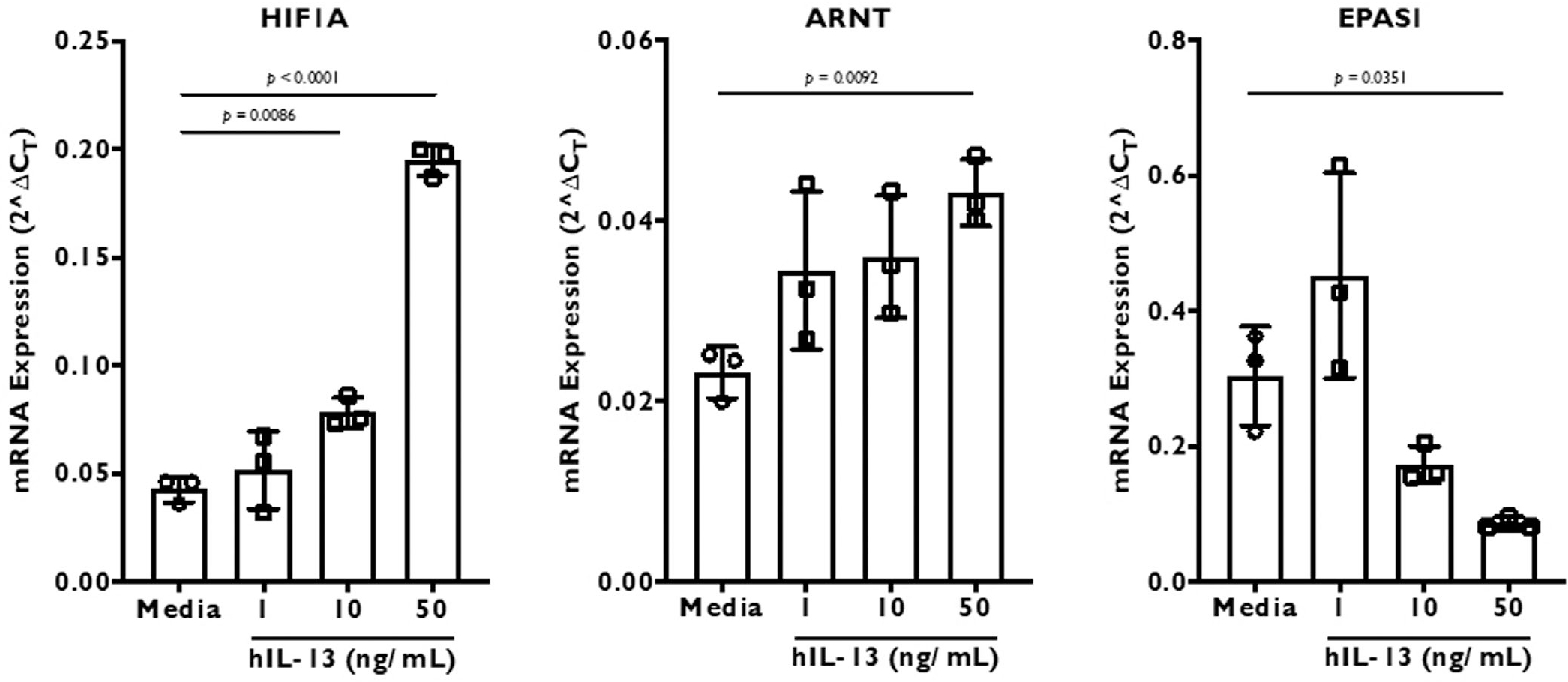
The effect of 3 different doses—1, 10, and 50 ng/mL—of recombinant human IL-13 on HIF gene (HIF1A, ARNT, and EPAS1) expression in differentiated ALI culture cells. The culture cells were stimulated for 2 weeks before RNA extraction and real-time PCR. The results are represented as 2ΔCT, where ΔCT was calculated by normalizing target gene CT value to β-actin housekeeping gene. Data are represented as mean ± SD. Statistics was calculated using 1-way ANOVA with Dunnett’s multiple comparisons test. Significance was set at p < 0.05. ALI = air-liquid interface; ANOVA = analysis of variance; CT = threshold cycle; HIF = hypoxia-inducible factor; IL = interleukin; PCR = polymerase chain reaction; SD = standard deviation.
The kinetics of the IL-13 effect showed a similar pattern for HIF1A and ARNT gene expression, with peak expression on day 5 followed by relatively constant elevated level of gene expression. On the other hand, EPAS1 expression is downregulated within a day of treatment and the expression does not revert to starting level over the tested time period (Fig. 4).
FIGURE 4.
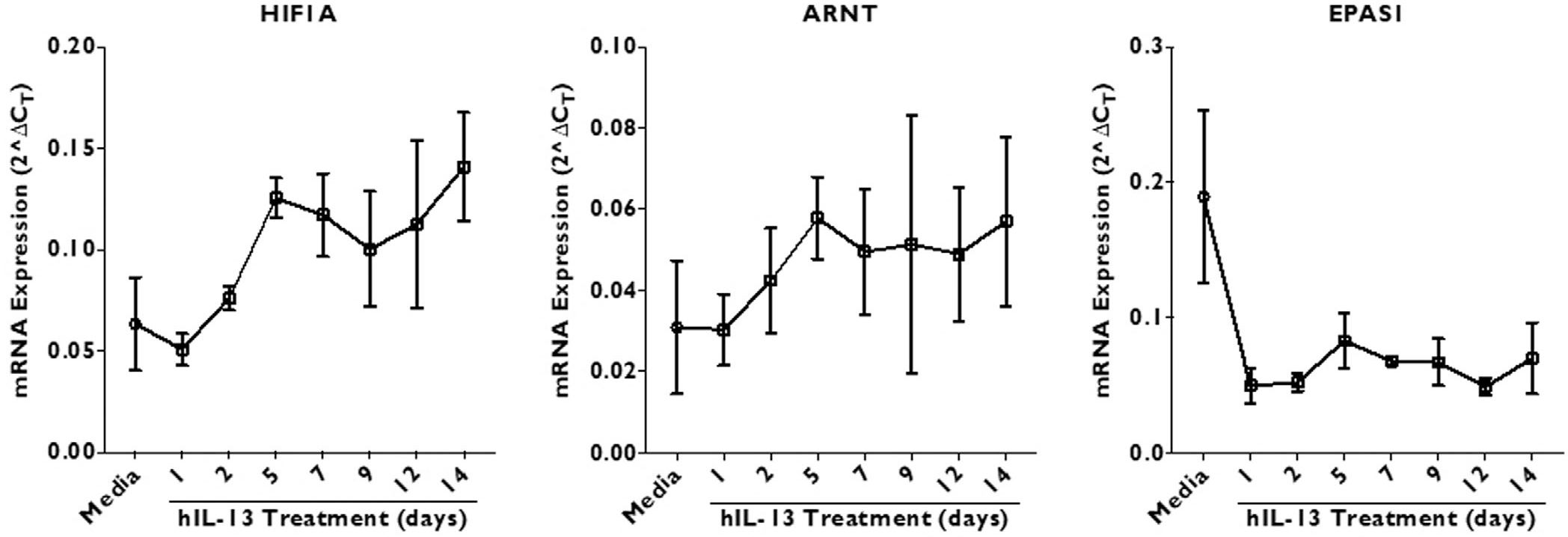
The effect of recombinant human IL-13 (50 ng/mL) on HIF gene (HIF1A, ARNT, and EPAS1) expression in cultured ALI cells. The samples were collected for RNA extraction on days 1, 2, 5, 7, 9, 12, and 14 post-stimulation. The results are represented as 2ΔCT, where ΔCT was calculated by normalizing target gene CT value to β-actin housekeeping gene. Data are represented as mean ± SD. Statistics was calculated using 2-way ANOVA with multiple comparisons test. Significance was set at p < 0.05. ALI = air-liquid interface; ANOVA = analysis of variance; CT = threshold cycle; HIF = hypoxia-inducible factor; IL = interleukin; SD = standard deviation.
CD73 surface expression on IL-13 stimulated cells
Ecto-5′-nucleotidase (CD73) is a surface glycoprotein which assists in conversion of danger signals ATP, ADP, and AMP into anti-inflammatory adenosine. CD73 can be regulated by HIF-1α and functions to protect the epithelial barrier during hypoxia.7 We employed flow cytometry analysis to measure CD73 expression over 2 weeks of IL13 treatment of sinonasal epithelial cells (Fig. 5). CD73 expression was significantly upregulated on day 5 and 7, an observation that was remarkably similar to HIF1A gene expression. Although HIF1A expression stabilized from days 5 to 14, CD73 surface expression reverted back to starting level by day 14.
FIGURE 5.
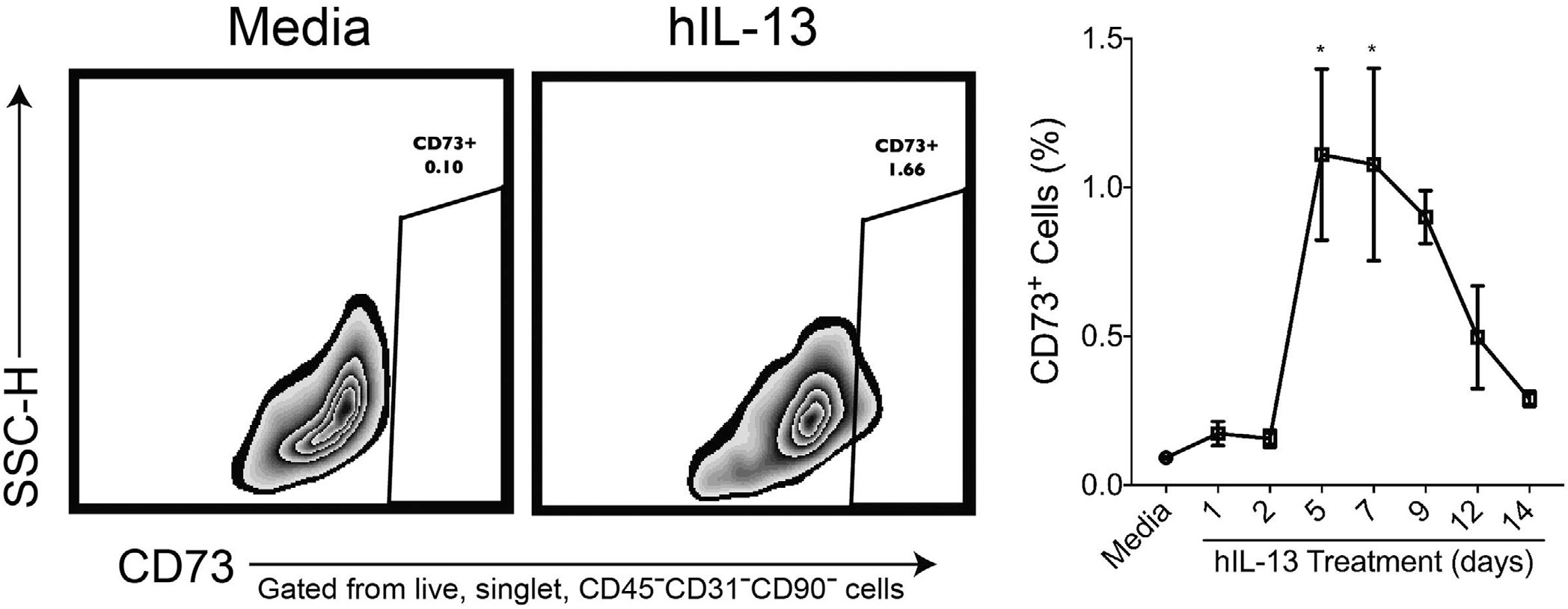
CD73 surface expression post–IL-13 stimulation of cultured human ALI cells. Three replicates each of IL-13 (50 ng/mL) stimulated cells were harvested at days 1, 2, 5, 7, 9, 12, and 14 followed by flow cytometry. (A) Representative flow plots CD73 expression in media-treated (left panel) and IL-13-treated (right panel) cell cultures. Cells were gated based on size (FSC-A) vs granularity (SSC-A) followed by singlets (FSC-H vs FSC-W and SSC-H vs SSC-W). Live cells were then gated for by excluding cells expressing Zombie Aqua dye. CD45−CD31−CD90− cells were gated out to ensure only epithelial cells were included in the analysis of CD73+ cells. Data are represented as mean ± SEM. Statistics was calculated using 2-way ANOVA with multiple comparisons test. Significance was set at p < 0.05. ALI = air-liquid interface; ANOVA = analysis of variance; FSC-A = forward light scatter area; FSC-H = forward light scatter height; FSC-W = forward light scatter width; IL = interleukin; SEM = standard error; SSC-A = side scatter area; SSC-H = side scatter height; SSC-W = side scatter width.
In summary, our results indicate that IL-13 can alter hypoxia-related genes in a non-hypoxic manner which then upregulates an anti-inflammatory protein, CD73.
Discussion
In this study, we sought to determine the effect of IL-13 on the epithelial cell hypoxia signaling pathway and its downstream consequences. Consistent with previous findings,10,11,17 we observed that the major hypoxia-related gene, HIF1A, was upregulated in the sinonasal epithelial tissue from CRSwNP patients compared to control subjects. However, to the best of our knowledge, this is the first report of expression of all known HIFα (HIF1A, EPAS1, HIF3A) and HIFβ (ARNT, ARNT2) genes. We focused on the expression of the most well-studied and understood genes, HIF1A, EPAS1, and ARNT.
Several mechanisms of HIF activation have been described in the literature, from reduced oxygen supply to increased oxygen consumption by the microbiome (reviewed in Colgan et al.18). Additionally, cytokines, such as IL-1, IL-6, and TNF, can cause HIF-1α transcription in intestinal epithelium in a non-hypoxic manner.14 We hypothesized that IL-13, the major type 2 immune cytokine in CRS, might also affect the HIF gene expression. Therefore, we evaluated the hypoxia-related genes after an extended, 2-week exposure of fully differentiated ALI culture cells to IL-13. In Figure 2, we report the results of 4 independent experiments with 2 to 4 replicates in each experiment. Our results compare favorably to the data from whole tissue gene expression analysis (Fig. 1) and confirm that IL-13 can activate HIF gene expression in the sinonasal epithelium in a non-hypoxic manner. Finally, we report opposing effects of IL-13 on the 2 HIFα genes, HIF1A and EPAS1. This observation may relate to similarly opposing roles reported for myeloid HIF1A (upregulation) and EPAS1 (downregulation) in eosinophilia.16
To investigate whether IL-13 has a direct effect on hypoxia signaling, we performed a dose-response experiment with 1, 10, and 50 ng/mL of IL-13. The increasing intensity of HIF1A and ARNT expression suggests that the effect of IL-13 on HIF genes is likely direct. However, the precise pathway of IL-13–mediated HIF gene expression requires further investigation. The dose-response experiment (Fig. 3) together with the time-course experiment (Fig. 4) further confirm the activation of HIF1A and inhibition of EPAS1 gene expression by IL-13.
These results suggest questions for future investigation. For example, does IL-4, a prototypical Th2 cytokine closely related to IL-13, activate HIF signaling pathway in the respiratory epithelium? Also, what effect would hypoxic stress conditions have on IL-13/IL-4 modulation of the HIF pathway in the sinonasal epithelium?
Hypoxia and the expression of hypoxia signaling pathway genes has long been appreciated to be proinflammatory in nature. However, several recent studies have shown that hypoxia can have immunosuppressive effects through lactate induction (reviewed in Ivashkiv19). Whether lactate plays a role in tempering inflammation in sinonasal epithelium has not been studied and remains an active area of interest to us. However, our results show that the IL-13–induced effect on HIF genes promotes another immunosuppressive pathway in sinonasal epithelial cell culture. Together with CD39, CD73 has been reported to have anti-inflammatory effects through dephosphorylation of the danger signal ATP through ADP and AMP to increased adenosine production.7,20,21 Indeed, increased adenosine in extracellular space is known to dampen inflammation.22 After initial increase, surface expression of CD73 reduced to the baseline level by day 14 (Figs.5 and 6). The kinetics of CD73 expression suggests that a HIF-1α–mediated increase during acute inflammation is followed by HIF-independent suppression in chronic inflammation. The role of the CD73 inhibitory pathway in chronic sinonasal inflammation is an area for future investigation.
FIGURE 6.
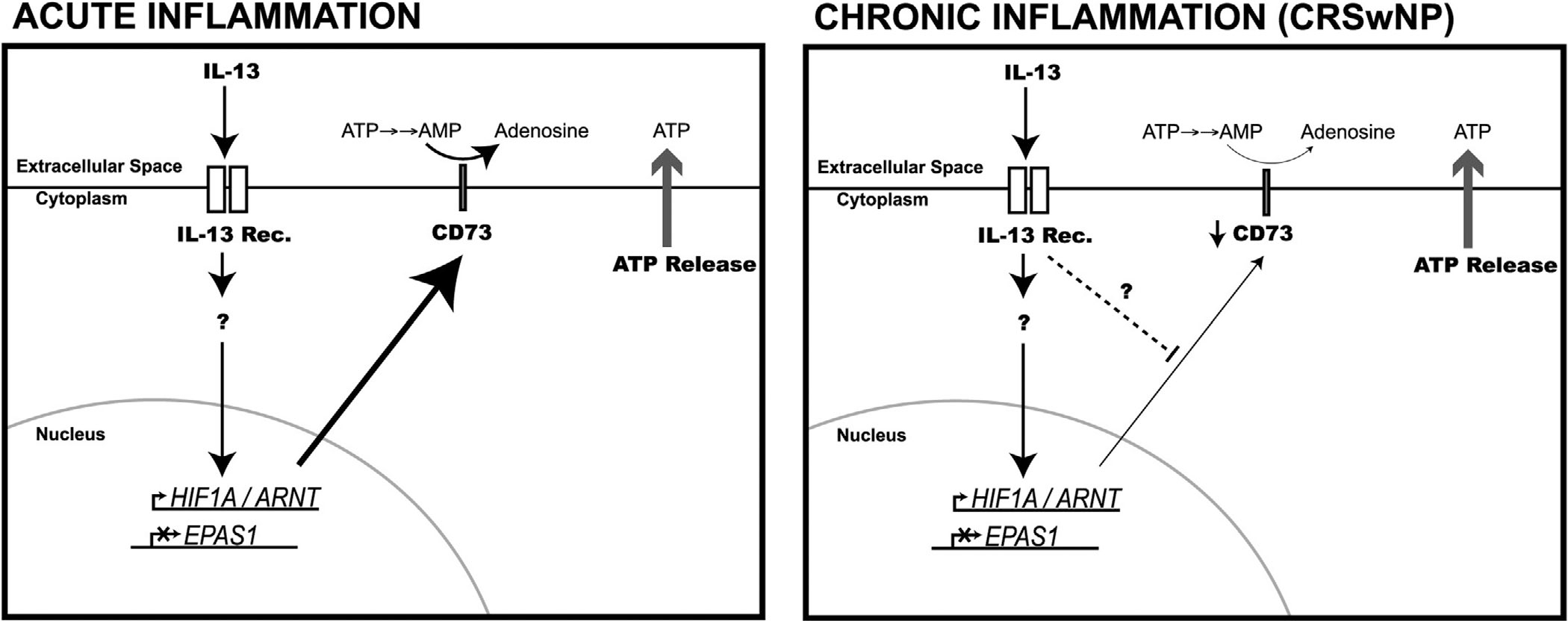
Schematic representation of the acute and chronic effect of IL-13 on the respiratory epithelium. Signaling through the IL-13 receptor promotes the expression of HIF1A and ARNT genes while also suppressing EPAS1 gene expression. Stabilized HIF-1α can promote CD73 gene expression and increased expression of CD73 on the cell surface. In differentiated culture, acute (up to day 7) IL-13 stimulation results in a significant increase in CD73 expression. Increased CD73 expression leads to metabolism of AMP to adenosine. On the other hand, with chronic exposure to IL-13 (>10 days), CD73 expression falls back to baseline levels through an unknown mechanism. The inhibition of CD73 is independent of continued HIF1A/ARNT expression or EPAS1 suppression. AMP = adenosine monophosphate; HIF = hypoxia-inducible factor; IL = interleukin.
Although the data presented in this study clearly indicate a role for IL-13 in the activation of the HIF signaling pathway, there are some limitations to consider. IL-13 can increase permeability by reducing E-cadherin in cultures and changing the epithelial cell population through epithelialto-mesenchymal transition.4 Our studies also revealed increased TP63 gene expression and basal cell population (by flow cytometry) after 2 week treatment with IL-13 (Khalil et al., unpublished observation). Whether these effects of IL-13 on differentiated sinonasal epithelial cells will affect their role in activating the HIF signaling pathway is currently unknown.
Conclusion
We report that IL-13 can activate or inhibit HIF gene expression in sinonasal epithelial cells in a non-hypoxic manner. Furthermore, IL-13–mediated stimulation of the hypoxia signaling pathway may have an anti-inflammatory effect mediated via CD73.
Funding sources for the study:
National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) (R01 AI132590 to A.P.L.) Potential conflict of interest: The authors declare no competing financial interests.
References
- 1.Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. [DOI] [PubMed] [Google Scholar]
- 2.Rudmik L Economics of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2017;17:20. [DOI] [PubMed] [Google Scholar]
- 3.Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469–479. [DOI] [PubMed] [Google Scholar]
- 4.Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poposki JA, Uzzaman A, Nagarkar DR, et al. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps” J Allergy Clin Immunol. 2011;128:73–81.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsune S, Kono M, Sun D, Ushikai M, Kurono Y. Hypoxia in paranasal sinuses of patients with chronic sinusitis with or without the complication of nasal allergy. Acta Otolaryngol. 2003;123:519–523. [DOI] [PubMed] [Google Scholar]
- 7.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152:598–609. [DOI] [PubMed] [Google Scholar]
- 9.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ, Cho HJ, Shin WC, Song HA, Yoon JH, Kim CH.Hypoxia-mediated mechanism of MUC5AC production in human nasal epithelia and its implication in rhinosinusitis. PLoS One. 2014;9:e98136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song HA, Kim YS, Cho HJ, et al. Hypoxia modulates epithelial permeability via regulation of vascular endothelial growth factor in airway epithelia. Am J Respir Cell Mol Biol. 2017;57:527–535. [DOI] [PubMed] [Google Scholar]
- 12.Min HJ, Kim TH, Yoon JH, Kim CH.Hypoxia increases epithelial permeability in human nasal epithelia. Yonsei Med J. 2015;56:825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin J, Chang DY, Kim SH, et al. Role of hypoxia-inducible factor-1α expression in regulatory T cells on nasal polypogenesis. Laryngoscope. 2014;124:E151–E159. [DOI] [PubMed] [Google Scholar]
- 14.Hellwig-Bürgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1β and tumor necrosis factor-α stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- 15.Cheng KJ, Bao YY, Zhou SH. The role of hypoxia inducible factor in nasal inflammations. Eur Rev Med Pharmacol Sci. 2016;20:5067–5076. [PubMed] [Google Scholar]
- 16.Crotty Alexander LE, Akong-Moore K, Feldstein S, et al. Myeloid cell HIF-1α regulates asthma airway resistance and eosinophil function. J Mol Med. 2013;91:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho HJ, Kim CH. Oxygen matters: hypoxia as a pathogenic mechanism in rhinosinusitis. BMB Rep. 2018;51:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colgan SP, Furuta GT, Taylor CT. Hypoxia and innate immunity: keeping up with the HIFsters. Annu Rev Immunol. 2020;38:341–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivashkiv LB. The hypoxia–lactate axis tempers inflammation. Nat Rev Immunol. 2020;20:85–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eltzschig HK, Ibla JC, Furuta GT, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium. J Exp Med. 2003;198:783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eltzschig HK, Köhler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aherne CM, Collins CB, Rapp CR, et al. Coordination of ENT2-dependent adenosine transport and signaling dampens mucosal inflammation. JCI Insight. 2018;3:e121521. [DOI] [PMC free article] [PubMed] [Google Scholar]


