Abstract
Background:
There is no evidence of peptides-probiotics symbiosis as supplements in aquafeeds.
Aim:
To evaluate the effect of peptides and probiotics supplementation via diet on blood parameters and growth performance of juvenile Piaractus brachypomus, an Amazonian fish, during the growth-out phase.
Methods:
120 juvenile P. brachypomus (242.77 g) were placed into twelve 200-l tanks (10 fish/tank), housed in an indoor open system with constant water renovation (flow rate:1.50 l/minute). The experiment used a completely randomized design with a 4 × 5 factorial arrangement [4 doses of supplementation (CD: commercial diet; PepD: CD+1.50% of peptides per CD weight; ProD: CD+40.00 ml of activated probiotics per kg of diet (Lactobacillus spp., Rhodopseudomonas spp., Saccharomycetes spp.); PepProD: CD+Pep+Pro); 5 sampling times (zero, second, fourth, sixth, and eighth week); n = 3]. Fish were fed twice a day at a feeding rate of 1% of body weight. At each sampling time, blood was collected and fish were measured for growth performance analysis. Data were analyzed by using two-way ANOVA and Tukey’s test (p < 0.05).
Results:
The values of hematocrit (18.31%), leukocytes (1,216.67 mm3), neutrophils (81.27%), lymphocytes (18.73%), albumin (1.08 g/dl), relative growth rate (1.002%/day), and the Fulton allometric condition factor (2.03) remained constant throughout the experiment (p > 0.05). Plasma glucose decreased for all fish in the second week (59.56 mg/dl); then, that level increased in fish fed with the CD (89.00 mg/dl), while fish fed with PepD, ProD, and PepProD showed constant values (57.22 mg/dl). The plasma protein levels were constant in fish fed with the PepD and PepProD, (p > 0.05), while fish fed with the CD and ProD showed non-constant and higher values. At the end of the trial, fish fed with the PepProD showed the highest weight gain and the lowest feed conversion rate (39.66 g; 0.97).
Conclusion:
It is possible to maintain the stability of plasma glucose and plasma protein by supplementing diets with peptides, but the peptides-probiotics symbiosis administrated via diet contributes to maintaining the stability of plasma glucose and plasma protein and to improve the growth performance of juvenile P. brachypomus during the growth-out phase.
Keywords: Amazonian fish farming, Neotropical fish, Paco, Plasma glucose, Plasma protein
Introduction
Piaractus brachypomus is a native species from the Amazon region distributed across of Ecuador, Colombia, Peru, and Brazil (Escobar et al., 2019). It is an omnivorous fish (Guimarães and Martins, 2015; Angeles-Escobar et al., 2021), accepts balanced extruded diets (Baldisserotto, 2013; Angeles-Escobar et al., 2021), and is the main native fish produced by aquaculture in Peru (Angeles-Escobar et al., 2021; PRODUCE, 2023). Its docility and rusticity, resistance to handling and common diseases in aquaculture production systems (Angeles-Escobar et al., 2021), and tolerance to wide physicochemical ranges in water (Ríos, 2021) are some characteristics that favor its production. Nevertheless, better efforts must be made to improve the productivity of farming in intensive systems.
Numerous studies have shown that peptides supplementation via diet improves fish growth and health as happened in some marine fish (Hevrøy et al., 2005; Kotzamanis et al., 2007; Tang et al., 2008), continental fish (Li et al., 2020; López-Macías and Salas-Benavides, 2020; Costa et al., 2022) and Amazonian fish, such as in Pseudoplatystoma punctifer, where the inclusion level of 1.50% of peptides in aquafeeds improves the growth performance in the species (Cortegano et al., 2022). Peptides are heteropolymers composed of amino acid residues, which present high digestibility in comparison with free amino acids (NRC, 2011; Baldisserotto, 2013; Cortegano et al., 2022), capacity to secrete digestive enzymes optimizing food utilization into gut (Rønnestad et al., 2017; Guzmán-Quimbayo et al., 2022), and once absorbed by the enterocytes, are distributed to different tissues to develop metabolic functions required by the animal, including the formation of antimicrobial, immunomodulatory and antioxidant peptides. Likewise, peptides are able to produce orexigenic components that stimulate or inhibit appetite in fish (Velasco et al., 2019).
Other experiences in aquaculture demonstrate the potential of dietary supplementation with probiotics (Chauhan and Singh, 2018; El-Saadony et al., 2021), since they are live beneficial bacteria introduced into the gastrointestinal tract that promote good health and fish growth by enhancing the internal microbial balance and could minimize antibiotic uses in aquaculture practices (Gupta et al., 2014; Chauhan and Singh, 2018; El-Saadony et al., 2021). The potential of some probiotics is well known, such as Bacillus velezensis and Lactobacillus sp., so they can express genes linked to the secretion of lipopeptides with antimicrobial activity, contributing to the health, evidenced by expressions of immunomodulatory agents or in the regulation of hematological parameters, and growth of the animal (Gómez and Balcázar, 2008; Yi et al., 2018; Akter et al., 2020). In Myleus schomburgkii, an Amazonian fish, the inclusion of 40 ml of activated probiotics per kg of diet (pool of Lactobacillus spp., Rhodopseudomonas spp., and Saccharomycetes spp.) has resulted in better growth performance for the species (Maldonado and Taricuarima, 2017).
There is no evidence of studies carried out that combined peptides and probiotics as supplements in aquafeeds, being this study is novel research for Amazonian aquaculture. Thus, this study aimed to evaluate the effect of peptides and probiotics supplementation via diet on blood parameters and growth performance of juvenile P. brachypomus during the growth-out phase.
Materials and Methods
Study place
The study was carried out at the Aquaculture Laboratory of the “Estación Experimental Pucallpa”, Instituto Veterinario de Investigaciones Tropicales y de Altura of the Universidad Nacional Mayor de San Marcos, Ucayali, Peru (8°38’32.9”S, 74°57’06.8”W).
Animals
One hundred twenty juvenile P. brachypomus were used in this study. The animals came from the same spawning and the own reproduction procedures developed in the study place and they were five and a half months old, weighed 242.77 ± 19.90 g, and lengthed 24.94 ± 2.98 cm.
Experimental design and treatments
Initially, all fish were placed into a 2,000-l tank housed in an indoor open system with constant water renovation (flow rate 2.50 l/minute), controlled aeration (4.46 ± 1.98 mg/l dissolved oxygen), temperature (25.36°C ± 0.52°C), and pH (7.46 ± 0.60) and 12 hours photoperiod. The fish were quarantined for 20 days to guarantee their welfare before the initiation of the feeding trial. During that period, the fish received sodium chloride baths (5 g/l once a day for the first week and 2 g/l three times per week for the following days) as a prophylactic procedure to maintain fish welfare (Cerdeira e t al., 2018). In addition, the fish were fed twice a day (at 08:00 and 16:00) until apparent satiation with a commercial diet with 26% crude protein available in the national market for the species (P. brachypomus and Colossoma macropomum) and it is in accordance with the recommendations on the nutritional requirement of the species according to farming experiences in conventional systems (Tafur-Gonzales et al., 2009; Guimarães and Martins, 2015; FONDEPES, 2017).
After that, juvenile P. brachypomus (242.77 ± 19.90 g; 24.94 ± 2.98 cm) were placed into twelve 200-l fiber glass tanks (10 fish/tank) (Gomes et al., 2006), housed in an indoor open system with constant water renovation (flow rate:1.50 l/minute), and 12 hours photoperiod. The experiment used a completely randomized design with a 4 × 5 factorial arrangement [4 doses of supplementation with peptides and probiotics via diet for the fish and 5 sampling times (zero, second, fourth, sixth, and eighth week of feeding)] and three replications each (n = 3). The doses of supplementation were as follows: 1) CD = commercial diet with 26% crude protein, 2) PepD = CD+1.50% of peptides per CD weight (Fish 40 AQUA NATURAL), 3) ProD = CD+40.00 ml of activated probiotics per kg of diet (pool of Lactobacillus spp., Rhodopseudomonas spp., and Saccharomycetes spp. activated by using 1,800 ml of water+100 ml of molasses+100 ml of BIOEM AQUA®, fermented in anaerobic conditions for 7 days and maintained under 15°C until use) and, 4) PepProD = CD+1.50% of peptides per CD weight+40.00 ml of activated probiotics per kg of diet. Peptides and probiotics were added to the diet in every meal by aspersion.
During the experimental period, all fish were fed twice a day (at 08:00 and 16:00) at a feeding rate of 1% of body weight for 8 weeks. The water quality parameters, dissolved oxygen (4.73 ± 0.11 mg/l), temperature (25.02°C ± 0.60°C), and pH (7.74 ± 0.10), were daily monitored (HANNA, model HI9819) and maintained within the comfort range for the species (Ríos, 2021).
Blood parameters
At zero (as basal sample), second, fourth, sixth, and eighth week of the trial, blood was collected from one previously anesthetized (1.50 ml/l of eugenol) fish, randomly captured per tank, via caudal vessel puncture using 10% EDTA solution as the anticoagulant (Dos Santos et al., 2021). Blood was analyzed by hematocrit (Ht – %) using heparinized microhematocrit capillary tubes (Corning®), centrifuged at 900 rpm (X–3,012), and analyzed with an hematocrit reader (CRIPTOCAT); by white blood cells count in 1 mm3 (neutrophils – %, eosinophils – %, basophils – %, lymphocytes – %, and monocytes – %) according to De Oliveira et al. (2018). From the blood plasma, the following analyses were performed: glucose (mg/dl) via the enzymatic-colorimetric method (glucose oxidase); total proteins (g/dl) and albumin (g/dl) following the modified biuret method; all values were quantified by using an automated equipment (Mindray BS-240E; Mindray Bc-30). After each blood sampling time, the fish captured were measured for growth performance analyses, and baths with sodium chloride (5g/l) for 5 minutes were done (Cerdeira et al., 2018).
Growth performance
At zero, second, fourth, sixth, and eighth weeks of the experimental period, the fish were anesthetized by using eugenol (0.20 ml/l) (Dos Santos et al., 2021) to evaluate the growth performance in terms of final weight; weight gain [final weight – initial weight]; feed conversion rate [feed intake (g)/weight gain (g)]; relative growth rate [(eg−1) × 100; where “e” is the nepper number, g=(ln(final weight) – ln(initial weight))/(t2 – t1)], and Fulton’s allometric condition factor [weight/length3]. After each sampling time, the fish collected were bath with sodium chloride (5g/l) for 5 minutes (Cerdeira et al., 2018).
Statistical analysis
The initial homogeneity of fish weight was affirmed by Cochran’s Q test (p < 0.05). Normality was ascertained by the Shapiro–Wilk test (p < 0.05) and homoscedasticity by the Breusch–Pagan test (p < 0.05). To determine the repetitions (triplicate), the principle of reduction to the 3Rs in animal experimentation (replace, reduction, and refinement) was used, which allows us to affirm that three repetitions per treatment were appropriate to verify the statistical difference between the treatments (NRC, 2011). Analysis of the blood parameters data and growth performance data were performed by using two-way ANOVA and Tukey’s test (p < 0.05). The data were processed using the software Statistica 10.0.
Ethical approval
All experimental procedures were carried out according to guidelines set forth by CONCEA (2013) and Jenkins et al. (2014), and this study was approved by the Graduate School of Faculty of Veterinary Medicine at the Universidad Nacional Mayor de San Marcos, in accordance with the animal welfare protocols.
Results
Blood parameters
The values of hematocrit (18.31%), leukocytes (1,216.67 mm3), neutrophils (81.27%), and lymphocytes (18.73%) remained constant throughout the experiment (p > 0.05) (Table 1). Eosinophil, basophil, and monocyte cells were not observed.
Table 1. Hematological and biochemical parameters of juvenile P. brachypomus supplemented with peptides and probiotics via diet.
| Parameters | Diet | Weeks of feeding | Pooled standard error | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | D | T | D×T | |||
| Hematocrit (%) | CD | 16.50 | 13.00 | 21.33 | 22.00 | 17.00 | 3.185 | n.s | n.s | n.s |
| PepD | 15.67 | 15.00 | 19.33 | 17.00 | ||||||
| ProD | 19.00 | 18.00 | 24.00 | 22.33 | ||||||
| PepProD | 17.00 | 17.67 | 16.33 | 18.33 | ||||||
| Leucocytes (mm3) | CD | 1341.67 | 1,116.67 | 1066.67 | 1,133.33 | 1,100.00 | 3.029 | n.s | n.s | n.s |
| PepD | 1,133.33 | 1,000.00 | 1,450.00 | 1,083.33 | ||||||
| ProD | 966.67 | 1,933.33 | 1,250.00 | 1,083.33 | ||||||
| PepProD | 1,266.67 | 1,100.00 | 1,550.00 | 1,233.33 | ||||||
| Neutrophils (%) | CD | 80.67 | 80.33 | 82.00 | 75.67 | 84.33 | 1.211 | n.s | n.s | n.s |
| PepD | 78.67 | 83.33 | 77.67 | 84.33 | ||||||
| ProD | 83.00 | 86.33 | 83.67 | 82.33 | ||||||
| PepProD | 78.00 | 79.33 | 80.33 | 81.00 | ||||||
| Lymphocytes (%) | CD | 19.33 | 19.67 | 18.00 | 24.33 | 15.67 | 1.211 | n.s | n.s | n.s |
| PepD | 21.33 | 16.67 | 22.33 | 15.67 | ||||||
| ProD | 17.00 | 13.67 | 16.33 | 17.67 | ||||||
| PepProD | 22.00 | 20.67 | 19.67 | 19.00 | ||||||
| Albumin (g/dl) | CD | 1.02 | 0.97 | 1.20 | 1.27 | 1.03 | 0.795 | n.s | n.s | n.s |
| PepD | 0.90 | 1.03 | 1.00 | 1.07 | PepD | |||||
| ProD | 1.37 | 1.13 | 1.13 | 1.13 | ProD | |||||
| PepProD | 1.03 | 1.00 | 0.97 | 1.00 | PepProD | |||||
Means of triplicate analyses by sample are showed. CD = Commercial diet – CD. PepD = CD+1.50% of peptides per CD weight (Fish 40 AQUA NATURAL). ProD = CD+40.00 ml of activated probiotics per kg of diet (BIOEM AQUA®). PepProD = CD+1.50% of peptides per CD weight+40.00 ml of activated probiotics per kg of diet. n.s = without statistical significance (p > 0.05). D = doses of supplementation of peptides and probiotics via diet for the fish, T = sampling time, D×T = interaction.
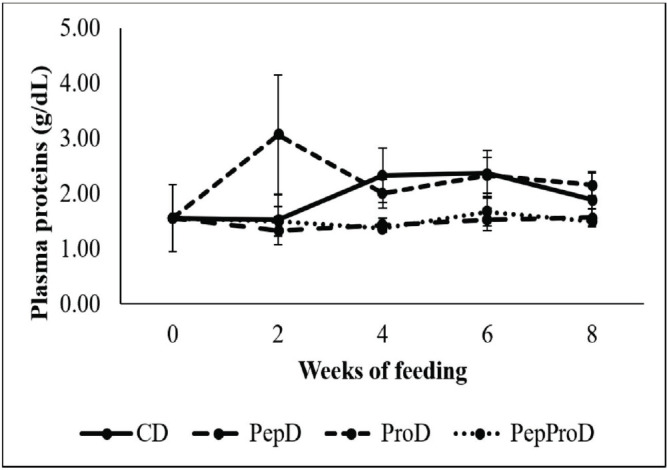
In relation to the analysis of blood biochemistry parameters, only plasma glucose showed an interaction between the factors analyzed, so for the other parameters the factors were analyzed separately. Plasma glucose (Fig. 1) decreased for all fish in the second week of feeding (59.56 mg/dl). After that, there was an increase in the level of plasma glucose in fish fed with the CD (89.00 mg/dl), while fish supplemented with peptides and probiotics or both via diet showed constant values since the second week (57.22 mg/dl). In fish fed with the PepD and PepProD, the plasma protein levels (Fig. 2) were always constant throughout the experiment (p > 0.05) and similar to the basal value (at week 0), but lower in comparison to the other groups. Fish fed with the ProD showed the highest plasma protein value in the second week of feeding, but then remained at similar values than fish fed with the CD. Finally, fish fed with the ProD, since the second week of feeding, and with the CD, since the fourth week of feeding, showed higher plasma protein levels than the basal reported. Plasma albumin values always remained constant (1.08 g/dl) throughout the 8 weeks of feeding (Table 1).
Fig. 1. Plasma glucose (mg/dl) of juvenile P. brachypomus supplemented with peptides and probiotics via diet. CD = Commercial diet – CD. PepD = CD+1.50% of peptides per CD weight (Fish 40 AQUA NATURAL). ProD = CD+40.00 ml of activated probiotics per kg of diet (BIOEM AQUA®). PepProD = CD+1.50% of peptides per CD weight+40.00 ml of activated probiotics per kg of diet. Vertical bars denote standard deviation.
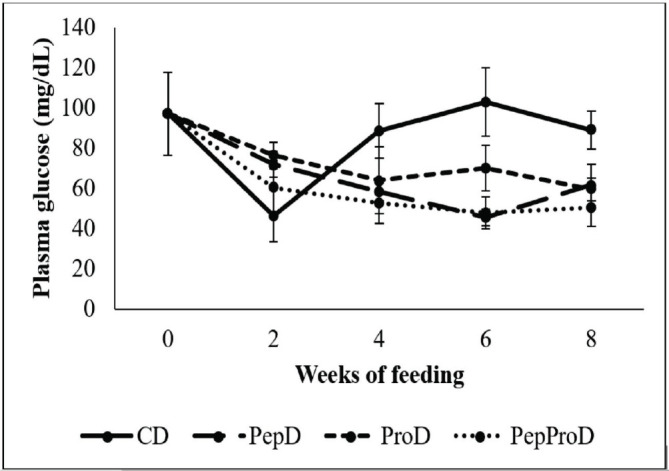
Fig. 2. Plasma protein (g/dl) of juvenile P. brachypomus supplemented with peptides and probiotics via diet. CD = Commercial diet – CD. PepD = CD+1.50% of peptides per CD weight (Fish 40 AQUA NATURAL). ProD = CD+40.00 ml of activated probiotics per kg of diet (BIOEM AQUA®). PepProD = CD+1.50% of peptides per CD weight+40.00 ml of activated probiotics per kg of diet. Vertical bars denote standard deviation.
Growth performance
None of the analyzed parameters showed interaction between the factors; therefore, the analyses were done separately for each factor (Table 2).
Table 2. Growth performance of juvenile P. brachypomus supplemented with peptides and probiotics via diet.
| Parameters | Diet | Weeks of feeding | Pooled standard error | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | D | T | D×T | |||
| Final weight (g) | CD | 242.90Aa | 265.85Bb | 264.04Ab | 265.61Ab | 267.45Ac | 5.054 | * | * | n.s |
| PepD | 242.50Aa | 251.05Ab | 268.77ABc | 273.20Bcd | 276.00Cd | |||||
| ProD | 242.58Aa | 252.08Ab | 266.90Ac | 268.30Acd | 270.00Bd | |||||
| PepProD | 243.10Aa | 266.50Bb | 272.30Bc | 272.15Bc | 282.43Dd | |||||
| Feed conversion rate | CD | - | 1.48Ba | 1.76Aa | 1.63Aa | 1.51Aa | 0.719 | * | * | n.s |
| PepD | - | 3.97Aa | 1.34Ab | 1.26Ab | 1.12Ab | |||||
| ProD | - | 3.57Aa | 1.45Ab | 1.45Ab | 1.37Ab | |||||
| PepProD | - | 1.45Ba | 1.28Aa | 1.31Aa | 0.97Bb | |||||
| Relative growth rate (%/day) | CD | - | 1.002 | 1.002 | 1.002 | 1.002 | 0.001 | n.s | n.s | n.s |
| PepD | - | 1.001 | 1.002 | 1.003 | 1.003 | |||||
| ProD | - | 1.002 | 1.002 | 1.002 | 1.002 | |||||
| PepProD | - | 1.002 | 1.003 | 1.003 | 1.004 | |||||
| Fulton’s allometric condition factor | CD | - | 1.80 | 1.80 | 1.90 | 2.00 | 0.001 | n.s | n.s | n.s |
| PepD | - | 1.90 | 2.20 | 2.10 | 2.40 | |||||
| ProD | - | 1.80 | 1.80 | 2.00 | 2.20 | |||||
| PepProD | - | 2.00 | 2.10 | 2.10 | 2.30 | |||||
Means of triplicate analyses by treatment are showed. No mortalities were reported. CD = Commercial diet – CD. PepD = CD+1.50% of peptides per CD weight (Fish 40 AQUA NATURAL). ProD = CD+40.00 ml of activated probiotics per kg of diet (BIOEM AQUA®). PepProD = CD+1.50% of peptides per CD weight+40.00 ml of activated probiotics per kg of diet. n.s = without statistical significance (p > 0.05). As the interactions were not significant (p > 0.05), factors were analyzed separately; in those cases, different capital letters indicate that there is a statistically significant difference between the doses of supplementation of peptides and probiotics via diet for each sampling time (p < 0.05), while different lower case letters indicate that there is a statistically significant difference between the sampling time for each type of supplementation of peptides and probiotics via diet (p < 0.05). D = doses of supplementation of peptides and probiotics via diet for the fish, T = sampling time, D×T = interaction.
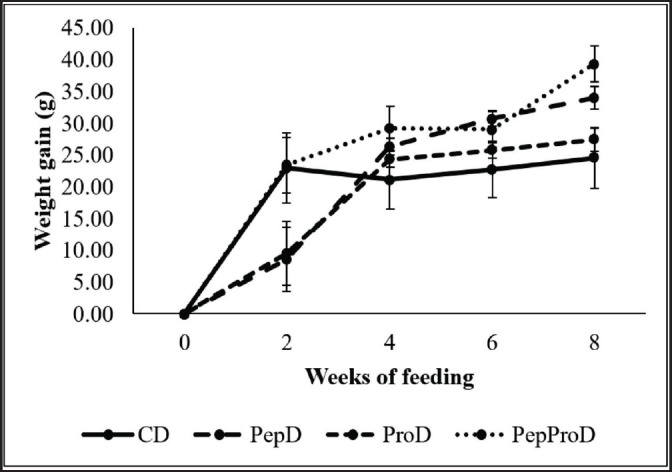
The fish began the experiment with 242.77 g showing differentiated performance at the second week of feeding with higher weight gains in fish fed with the CD and PepProD compared to fish fed with the PepD and ProD. After that, all fish gained weight in the fourth and sixth weeks, without exhibiting differences between the groups of fish. At the end of the trial, fish fed the PepProD showed the highest weight gain, followed by fish fed with the PepD. The lower weight gain occurred in fish fed with the ProD, as well as in fish fed with the CD (Fig. 3).
Fig. 3. Weight gain (g) of juvenile P. brachypomus supplemented with peptides and probiotics via diet. CD = Commercial diet – CD. PepD = CD+1.50% of peptides per CD weight (Fish 40 AQUA NATURAL). ProD = CD+40.00 ml of activated probiotics per kg of diet (BIOEM AQUA®). PepProD = CD+1.50% of peptides per CD weight+40.00 ml of activated probiotics per kg of diet. Vertical bars denote standard deviation.
In relation to feed conversion, the highest values were reported in the second week of feeding for fish fed with the PepD (3.97) and ProD (3.57); except for those values, the feed conversion values resulted in the experiment remained between 1.12 and 1.76, although at the eighth week of feeding the lowest feed conversion value (0.97) was obtained in juvenile Piaractus brachypomus fed with the PepProD.
The relative growth rate (1.002%/day) and the Fulton allometric condition factor (2.03) remained constant and without difference throughout the 8 weeks of experimentation.
Discussion
In this study, no alteration was observed in the hematological parameters in terms of hematocrit content and white cell count, which allows us to interpret that the supply of peptides and probiotics as supplements via diet, in the administrated doses used in this study, would have a neutral effect on hematological parameters for juvenile P. brachypomus. Some previous research about dietary supplementation with peptides and probiotics in fish coincided with our results, thus demonstrating their safe application as feedstuffs in diets for this species (Najim et al., 2014; Chauhan and Singh, 2018; Davies et al., 2020; El-Saadony et al., 2021; Costa et al., 2022).
The resulting hematocrit value in our research, which reflects the proportion of erythrocytes in the blood in relation to the amount of leukocytes, thrombocytes, and blood plasma (Ranzani-Paiva et al., 2013), was comparatively lower than that reported for juvenile Piaractus brachypomus raised under biofloc system technology (34.7%) (Angeles-Escobar et al., 2021) or exposed to hypoxia (37.33%) (Corredor-Castillo and Landines-Parra, 2018). In those cases, it is possible that the exposure to suspended solids or nitrite in biofloc system, or the exposure to a stressor agent such as hypoxia, has led to an increase in hematocrit values as a compensation in the fish to maintain an adequate oxygen transport for its metabolic functions and to maintain homeostasis (Corredor-Castillo and Landines-Parra, 2018; Angeles-Escobar et al., 2021). Nevertheless, although this species is capable of efficiently adjusting its hematological and biochemical responses according to environmental or farming conditions (Favero et al., 2022), the hematocrit values registered in this trial were in accordance with some commercial farming experiences under proper management of P. brachypomus (Acosta et al., 2017) and closed to that reported in Ranzani-Paiva et al. (1998) for characids.
The values of the white cells, which are related to the fish’s defense, were always constant during the development of this research, thus indicating similar physiological conditions in all fish. These values were close to that reported for juvenile P. brachypomus farmed in floating cages installed into a lake in the Ucayali region in Peru (Acosta et al., 2017), but lower than those found for C. macropomum, an Amazonian Serrasalmidae, produced in ponds (Grande-Fernández et al., 2023). However, hematological parameters in fish could be influenced by external factors, such as the environment, quality and quantity of the aquafeed supplied to fish, the farming system, and internal factors, such as the species, age, reproductive stage, and stress (Lochmann et al., 2009; Rodrigues, 2018; Corredor-Castillo and Landines-Parra, 2018). According to Ranzani-Paiva et al. (2013), the white cells most present in the blood are neutrophils and lymphocytes, and it is rare to observe eosinophil, basophil, and monocyte cells. For that, eosinophil, basophil, and monocyte cells were not observed in the blood samples. Furthermore, before the beginning of this trial, the absence of ectoparasites and endoparasites was verified in the fish, so that responds to the absence of eosinophilic cells too, since the presence of those cells is related to degrees of parasitism in the animal (Ranzani-Paiva et al., 2013).
The blood biochemical parameters are efficient indicators of fish’s welfare, since the increasing content of these parameters in the blood plasma, mainly, results from immediate and/or chronic exposure to stress conditions as a physiological strategy to maintain the homeostatic balance in fish (Baldisserotto, 2013; Angeles-Escobar et al., 2021). In this study, the plasma albumin was always constant but differences were found in plasma glucose and plasma proteins during the trial. The plasma glucose content of all fish showed a decrease in the second week of feeding in comparison to the basal value, probably as an adaptation response to the fish transference to the experimental units. However, as the feeding period elapsed, the plasma glucose content in fish fed with the supplemented diets (PepD, ProD, and PepProD) remained constant until the end of the trial, while fish feed with the CD increased their plasma glucose content again since the fourth week of feeding. In that sense, confinement conditions can affect different physiological processes in fish, such as their immune response, feeding and reproduction, and routes connected through stress activation pathways. The stimuli that generate different stress conditions are initially perceived by sensors in the hypothalamus, where two major regulatory axes are stimulated: the brain-sympathetic-chromaffin axis and the hypothalamic-pituitary-interrenal axis (HPI) (Fabbri et al., 1998; Pijanowski et al., 2015; Guzmán-Quimbayo et al., 2022). The peptides consumed via diet, once absorbed by the enterocytes, are distributed to different tissues to develop metabolic functions required by the fish, including the generation of neuropeptides that play leading roles in the HPI axis, modulating stress responses and contributing in the maintenance of homeostasis in fish, and the synthesis of insulin, a peptide that modulates the plasma glucose content (Khansari et al., 2017; Guzmán-Quimbayo et al., 2022). On the other hand, although probiotics consumed via diet or added in the water farming system are not directed to the synthesis of modulating components in response to stress, these microorganisms contribute to strengthening the immune response and resistance to pathogens in fish (Chauhan and Singh, 2018; El-Saadony et al., 2021). Therefore, it is likely that these functional characteristics associated with the consumption of peptides and probiotics, independently or in symbiosis, would have a regulatory effect to maintain the plasma glucose balance in juvenile P. brachypomus (Ocampo and Camberos, 1999; Barandica and Tort, 2008). Nonetheless, regarding the plasma protein content, the CD and the dietary supplementation with only probiotics were not enough to keep constant the plasma protein content in juvenile P. brachypomus in comparison with fish fed with diets supplemented with peptides. It is probably that the leaching of the supplement “probiotics” due to its liquid consistency and/or the use of non-indigenously probiotics (Lactobacillus spp., Rhodopseudomonas spp., and Saccharomycetes spp.) from the host (Puello-Caballero et al., 2018, Castañeda-Monsalve et al., 2019) did not favored the functional effects of probiotics as a supplement, although in the last case, El-Saadony et al. (2021) indicate a positive effect on the fish welfare by feeding supplemented diets with indigenous or exogenous probiotics. For that reason, we suggest that the regulatory effect on blood biochemical parameters is mainly related to the consumption of diets supplemented with peptides, although advantages of the peptides-probiotics symbiosis as supplements were observed for the growth performance in juvenile P. brachypomus in this study.
Although lower weight gains were observed in fish fed with PepD and ProD in the second week of feeding, probably due to changes in the food palatability (Cortegano et al., 2019) and no alterations were observed in the relative growth rate values during the experiment, at the end of the trial the highest final weight and weight gain resulted in fish fed with the PepProD, followed by fish fed with PepD, while fish fed with ProD and CD showed the lowest weight gain. Dietary peptides allow the formation of antimicrobial peptides and are precursors of immunomodulatory and antioxidant components promoting better growth performances in fish (Martínez-Alvarez et al., 2015; Halim et al., 2016). Likewise, its high digestibility in comparison with free amino acids (NRC, 2011; Baldisserotto, 2013; Cortegano et al., 2022) and its capacity to secrete digestive enzymes (Rønnestad et al., 2017; Guzmán-Quimbayo et al., 2022) improve fish growth as happened in some marine (Hevrøy et al., 2005; Kotzamanis et al., 2007; Tang et al., 2008) and continental fish (López-Macías and Salas-Benavides, 2020; Cortegano et al., 2022; Costa et al., 2022). These reasons support our results in fish-fed diets containing peptides as supplements. On the other hand, despite the functional effects associated with the consumption of probiotics such as improving growth performances, disease resistance, immunity, health status, intestinal epithelial barrier integrity, gut microbiome, and water quality (Gupta et al., 2014; Maldonado and Taricuarima, 2017; Chauhan and Singh, 2018; Carcelén et al., 2021; El-Saadony et al., 2021), as mentioned before, it is possible that the leaching of the supplement limited its biological effects. However, it is likely that both supplements supplied in symbiosis achieved better final weight and weight gain in juvenile P. brachypomus due to the viscosity of the product containing peptides, limiting the probiotics’ leaching and allowing joint beneficial action for the fish.
The feed conversion rate in juvenile P. brachypomus in this study remained between 1.12 and 1.76 and are in accordance with ranges naturally observed in commercial farming of this species (Angeles-Escobar et al., 2021; Favero et al., 2021). However, high feed conversion values were observed in the second week for fish fed with PepD and ProD, possibly as a response to the modification in the food’ palatability (Cortegano et al., 2019). At the end of the trial, the fish fed with the PepProD showed the lowest feed conversion (0.97). This result is very attractive, since the feed conversion value finally obtained is below to that reported for commercial farming of the species under adequate aquaculture practices, as well as for other species with similar corporal conditions, such as in C. macropomum (Grande-Fernández et al., 2023) and Piaractus mesopotamicus (Fernandes et al., 2000).
The values of Fulton’s allometric condition factor, which estimates the overall condition of fish welfare (Lima‐Júnior et al., 2002; Cortegano et al., 2019), suggest that the peptides and probiotics supplementation via diet do not affect fish welfare, as evidenced by the absence of mortality during the trial.
Conclusion
In conclusion, peptides administrated via diet contribute to maintaining the stability of plasma glucose and plasma protein contents in the blood plasma, but the peptide-probiotic symbiosis administrated via diet contributes to maintaining the stability of plasma glucose and plasma protein contents in the blood plasma and to improve the growth performance of juvenile P. brachypomus.
There is no evidence of carrying out studies that combined peptides and probiotics as supplements in aquafeeds, being this study a novel research for aquaculture with an Amazonian native species. However, we suggest expanding research by combining peptides and probiotics as a supplement in aquafeeds in longer feeding times, different farming systems, evaluating feeding costs, and validating these experiences on a larger production scale.
Acknowledgments
The authors are indebted to Universidad Nacional Mayor de San Marcos – RR N° 005753-2021-R/UNMSM (Project N° A21081351) for the financial support.
Conflict of interest
The authors declare that there is no conflict of interest.
Author’s contributions
All authors conceived the study, wrote the paper, and participated in monitoring the results. All authors read and approved the final manuscript.
Funding
Universidad Nacional Mayor de San Marcos – RR N° 005753-2021-R/UNMSM (Project N° A21081351).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Acosta C.R.R, Paredes R.O, Panduro P.P.V, and Flores J.I.V. Efecto de la densidad de siembra en el desempeño productivo y parámetros hematológicos de juveniles de Piaractus brachypomus “paco”cultivados en jaulas flotantes en la laguna Yarinacocha. Cultura Viva Amazónica. 2017;2(2):2017. [Google Scholar]
- Akter N, Hashim R, Pham H.Q, Choi S.D, Lee J.H, and Rajagopal K. Lactobacillus acidophilus antimicrobial peptide is antagonistic to Aeromonas hydrophila. Front. microbiol. 2020;11:570851. doi: 10.3389/fmicb.2020.570851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles-Escobar B.E, da Silva S.M.B, and Severi W. Growth, red blood cells, and gill alterations of red pacu (Piaractus brachypomus) fingerlings by chronic exposure to different total suspended solids in biofloc. J. World Aquac. Soc. 2021;53(3):652–668. [Google Scholar]
- Baldisserotto B. 3ra. Santa María, Brasil: Ed UFSM; 2013. Fisiologia de peixes aplicada à piscicultura. [Google Scholar]
- Barandica L.M.C, and Tort L.B. Neuroendocrinología e inmunología de la respuesta al estrés en peces. Rev Acad Colomb Cienc. 2008;32(123):267–284. [Google Scholar]
- Carcelén F, López M, San Martín F, Ara M, Bezada S, Ruiz-García L, Sandoval-Monzón R, López S, and Guevara J. Effect of probiotics administration at different levels on the productive parameters of guinea pigs for fattening (Cavia porcellus) Open Vet. J. 2021;11(2):222–227. doi: 10.5455/OVJ.2021.v11.i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda-Monsalve V.A, Junca H, García-Bonilla E, Montoya-Campuzano O.I.M, and Moreno-Herrera C.X. Characterization of the gastrointestinal bacterial microbiome of farmed juvenile and adult white Cachama (Piaractus brachypomus) Aquaculture. 2019;512:734325. [Google Scholar]
- Cerdeira K.A, Souza K.J.N.S, Ferreira J.B, Zampar A, Ono E.A, Affonso E.G. Soybean meal in diets for juveniles of pirarucu. Bol. Inst. Pesca. 2018;44(3):e318. [Google Scholar]
- Chauhan A, and Singh R. Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis. 2018;77:99–113. [Google Scholar]
- CONCEA (National Council for the Control of Animal Experimentation) Ministério da Ciência, Tecnologia e Inovação (Brazilian Ministry of Science, Technology and Inovation)COBEA. Brasília, Brazil: CONCEA; 2013. Diretriz Brasileira Para o Cuidado e a Utilização de Animais Para Fins Científicos e Didáticos (DBCA – Brazilian Directive for the Care and Utilization of Animals for Scientific and Teaching Purposes – DBCA) [Google Scholar]
- Corredor-Castillo A, and Landines-Parra M. Parámetros productivos y hematológicos de Piaractus brachypomus suplementados con ácido ascórbico y sometidos a estrés por hipoxia. Orinoquia. 2018;22(1):48–56. [Google Scholar]
- Cortegano C.A.A, de Alcântara A.M, da Silva A.F, Epifânio C.M, Bentes S.P.C, dos Santos V.J, Visentainer J.V, and Gonçalves L.U. Finishing plant diet supplemented with microalgae meal increases the docosahexaenoic acid content in Colossoma macropomum flesh. Aquac. Res. 2019;50:1291–1299. [Google Scholar]
- Cortegano C.A.A, Núñez-Montúfar R.L, Wong-Bardales D, Prada-Rojas D, Villanueva-Chávez C.A, Contreras-Salazar G, Bezada-Quintana S.G, Carcelén-Cáceres F.D, López-Guerra S. Efecto de la inclusión en la dieta de hidrolizado proteico de pescado sobre el crecimiento corporal y composición proximal del músculo de doncella (Pseduplatystoma punctifer) Rev. de Inv. Vet. del Peru. 2022;33(5):e22232. [Google Scholar]
- Costa M.N.F, Furtado Y.I.C, Monteiro C.C, Brasiliense A.R.P, and Yoshioka E.T.O. Physiological responses of tambaqui (Colossoma macropomum) fed diets supplemented with silage from fish and vegetable residues. Braz. J. Biol. 2022;84:e255493. doi: 10.1590/1519-6984.255493. [DOI] [PubMed] [Google Scholar]
- Davies S.J, Guroy D, Hassaan M, El-Ajnaf S.M, and El-Haroun E. Evaluation of co-fermented apple-pomace, molasses and formic acid generated sardine-based fish silages as fishmeal substitutes in diets for juvenile European sea bass (Dicentrachus labrax) production. Aquaculture. 2020;521:735087. [Google Scholar]
- De Oliveira C.A.C, Oliveira F.C.M, Souto P.S.S. Estudo morfológico de células sanguíneas de Piaractus brachypomus (Cuvier, 1817) Pubvet. 2018;12(9):1–6. [Google Scholar]
- Dos Santos R.B, Izel-Silva J, Fugimura M.M.S, Suita S.M, Ono E.A, Affonso E.G. Growth performance and health of juvenile tambaqui, Colossoma macropomum, in a biofloc system at different stocking densities. Aqua. Res. 2021;52:3549–3559. [Google Scholar]
- El-Saadony M.T, Alagawany M, Patra A.K, Kar I, Tiwari R, Dawood M.A.O, Dhana K, and Abdel-Latif H.M.R. The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- Escobar M.D.L, Ota R.P, Machado-Allison A, Andrade-López J, Farias I.P, and Hrbek T. A new species of Piaractus (Characiformes: Serrasalmidae) from the Orinoco Basin with a redescription of Piaractus brachypomus. J. Fish Biol. 2019;95(2):411–427. doi: 10.1111/jfb.13990. [DOI] [PubMed] [Google Scholar]
- Fabbri E, Capuzzo A, Moon T. The role of circulating catecholamines in the regulation of fish metabolism: an overview. Comp Biochem Physiol C Toxicol Pharmacol. 1998;120(2):177–192. doi: 10.1016/s0742-8413(98)10017-8. [DOI] [PubMed] [Google Scholar]
- Favero G.C, dos Santos F.A.C, Júlio G.S.C, Pedras P.P.C, Ferreira A.L, Silva W.S, Ferreira N.S, Neves L.C, and Luz R.K. Effects of short feed restriction cycles in Piaractus brachypomus juveniles. Aquaculture. 2021;536:736465. [Google Scholar]
- Favero G.C, dos Santos F.A.C, Júlio G.S.C, Batista F.S, Bonifácio C.T, Torres I.F.A, Paranhos C.O, and Luz R.K. Effects of water temperature and feeding time on growth performance and physiological parameters of Piaractus brachypomus juveniles. Aquaculture. 2022;548(2):737716. [Google Scholar]
- Fernandes J.B.K, Carneiro D.J, and Sakomura N.K. Fontes e níveis de proteína bruta em dietas para alevinos de pacu (Piaractus mesopotamicus) Rev. Bras. Zootec. 2000;29(3):646–653. [Google Scholar]
- FONDEPES (Fondo Nacional de Desarrollo Pesquero) Lima, Perú: Ministerio de la Producción del Perú; 2017. Manual del cultivo de gamitana. [Google Scholar]
- Gómez G.D, and Balcázar J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol. 2008;52:145–154. doi: 10.1111/j.1574-695X.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Gomes L.C, Chagas E.C, Martins-Junior H, Roubach R, Ono E.A, Lorenço J.N.P. Cage culture of tambaqui (Colossoma macropomum) in a central Amazon floodplain lake. Aquaculture. 2006;253:374–384. [Google Scholar]
- Grande-Fernández B.C, Cortegano C.A.A, Chávez C.A.V, Goncalvez L.U, Quintana S.G.B, Cáceres F.D.C, and Guerra S.L. Crecimiento corporal, composición proximal del músculo y parámetros hematológicos de juveniles de Colossoma macropomum alimentados con una dieta exclusivamente vegetal en comparación con una dieta con bajo contenido de harina de pescado. Rev. Inv. Vet. Perú. 2023;34(2):e23524. [Google Scholar]
- Guimarães I.G, and Martins G.P. Nutritional requirement of two Amazonian aquaculture fish species, Colossoma macropomum (Cuvier, 1816) and Piaractus brachypomus (Cuvier, 1818): a mini review. J. Apli, Ichthyol. 2015;31:57–66. [Google Scholar]
- Gupta A, Gupta P, and Dhawan A. Dietary supplementation of probiotics affects growth, immune response and disease resistance of Cyprinus carpio fry. Fish Shellfish Immunol. 2014;41(2):113–119. doi: 10.1016/j.fsi.2014.08.023. [DOI] [PubMed] [Google Scholar]
- Guzmán-Quimbayo F.G, Santana-Sepúlveda P, Mercado-Vianco L, Jara-Gutiérrez C, Álvarez-Álvarez C, and Alvarado-Almonacid N. Temuco, Chile: Universidad Autónoma de Chile; 2022. Una mirada a los péptidos y su potencial aplicación en acuicultura de peces. [Google Scholar]
- Halim N.R.A, Yusof H.M, and Sarbon N.M. Functional and bioactive properties of fish protein hydolysates and peptides: a comprehensive review. Trends Food Sci. Technol. 2016;51:24–33. [Google Scholar]
- Hevrøy E.M, Espe M, Waacbø O.R, Sandnes K, Ruud M, Hemre G.-I. Nutrient utilization in Atlantic salmon (Salmo salar L.) fed increased levels of fish protein hydrolysate during a period of fast growth. Aquac. Nutr. 2005;11(4):301–313. [Google Scholar]
- Jenkins J.A, Bowser P.R, Bowker J.D, MacMillan J.R, Nickum J.G, Rose J.D, Sorensen P.W, Whitledge G.W, Bart Jr. H.L, Rachlin J.W, Warkentine B.E. American Fisheries Society, American Institute of Fisheries Research Biologists, and American Society of Ichthyologists and Herpetologists. Bethesda, MD: American Fisheries Society; 2014. Guidelines for the use of fishes in research. [Google Scholar]
- Khansari A, Balasch J, Reyes-lópez F, Tort L. Stressing the inflammatory network : immuno-endocrine responses to allostatic load in fish. J. Mar. Sci. Technol. 2017;1(2):856–862. [Google Scholar]
- Kotzamanis Y.P, Gisbert E, Gatesoupe F.J, Zambonino-Infante J, and Cahu C. Effects of different dietary levels for fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae. Comp. Biochem. Physiol. 2007;147(1):205–214. doi: 10.1016/j.cbpa.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Li X, Wei X, Guo X, Mi S, Hua X, Li N, and Yao J. Enhanced growth performance, muscle quality and liver health of largemouth bass (Micropterus salmoides) were related to dietary small peptides supplementation. Aquac. Nutr. 2020;00:1–9. [Google Scholar]
- Lima-Júnior S.E, Cardone I.B, and Goiten R. Determination of a method for calculation of allometric condition factor fish. Acta Sci. 2002;24(2):397–400. [Google Scholar]
- Lochmann R, Chen R, Chu-Koo F.W, Camargo W.N, Kohler C.C, and Kasper C. Effects of carbohydrate-rich alternative feedstuffs on growth, survival, body composition, hematology, and nonspecific immune response of black pacu, Colossoma macropomum, and Red Pacu, Piaractus brachypomus. J. World Aquac. Soc. 2009;40(1):33–44. [Google Scholar]
- López-Macías J, and Salas-Benavides J. Efecto de harina de hidrolizado de vísceras en el crecimiento de trucha arco-íris (Oncorhynchus mykiss) Rev. MVZ Córdoba. 2020;26:e1989. [Google Scholar]
- Maldonado R, Taricuarima M. 2017. Tres niveles de inclusión del probiótico EM en el alimento sobre el crecimiento y composición corporal en alevinos de banda negra Myleus schomburgkii, (Serrasalmidae) cultivados en corrales. Graduate thesis, Universidad Nacional de la Amazonía Peruana, Iquitos, Peru. [Google Scholar]
- Martínez-Álvarez O, Chamorro S, and Brenes A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: a review. Int. Food Res. J. 2015;73:204–212. [Google Scholar]
- Najim S.M, Al-Noor S.S, and Jasim B.M. Effects of fish meal replacement with fish biosilage on some haematological and biochemical parameters in common carp Cyprinus carpio fingerlings. Int J Res Fish Aquac. 2014;4(3):112–116. [Google Scholar]
- NRC (National Research of Council) Washington DC: The National Academies Press; 2011. Nutrient requirements of fish and shrimp. [Google Scholar]
- Ocampo A, and Camberos L. Diagnóstico del estrés en peces. Veterinaria México. 1999;30(4):337–344. [Google Scholar]
- Pijanowski L, Jurecka P, Irnazarow I, Kepka M, Szwejser E, Verburg-van Kemenade B, Chadzinska M. Activity of the hypothalamus–pituitary–interrenal axis (HPI axis) and immune response in carp lines with different susceptibility to disease. Fish Physiol. Biochem. 2015;41(5):1261–1278. doi: 10.1007/s10695-015-0084-3. [DOI] [PubMed] [Google Scholar]
- PRODUCE (Ministerio de la Producción del Perú) Lima, Perú: Ministerio de la Producción del Perú; 2023. Anuario estadístico pesquero y acuícola 2022. [Google Scholar]
- Puello-Caballero L, Montaya-Campuzano O, Castañeda-Monsalve V, Moreno-Murillo L. Caracterización de la microbiota presente en el intestino de Piaractus brachypomus (Cachama blanca) Rev. Salud Anim. 2018;40(2):e02. [Google Scholar]
- Ranzani-Paiva M.J.T, Salles F.A, Eiras J.C, Das Eiras C.C, Ishikawa C.M, and Alexandrino A.C. Blood analysis of ‘‘curimbata’’ (Prochilodus scrofa), ‘‘pacu’’ (Piaractus mesopotamicus), and ‘‘tambaqui’’ (Colossoma macropomum), from Instituto de Pesca Experimental Stations, Sao Paulo, Brazil. Bol. Inst. Pesca. 1998;25:77–83. [Google Scholar]
- Ranzani-Paiva M.J.T, de Pádua S.B, Tavares-Dias M, Egami M.I. Maringá, Brasil: Editora da Universidade Estadual de Maringá; 2013. Métodos para análise hematológica em peixes. [Google Scholar]
- Ríos E.I. 1st. Lima, Peru: Editorial Barreto SAC; 2021. Calidad de Agua en el Cultivo de Organismos Acuáticos Amazónicos. [Google Scholar]
- Rodrigues A.L.G. 2018. Caracterização hematológica Da pirapitinga Piaractus brachypomus (Cuvier, 1818) em Condições de Cultivo, PhD. thesis, Universidade Federal do Acre, Rio Branco, Brazil. [Google Scholar]
- Rønnestad I, Gomes A, Murashita K, Angotzi R, Jönsson E, and Volkoff H. Appetite-controlling endocrine systems in teleosts. Front. Endocrinol. 2017;8:1–24. doi: 10.3389/fendo.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafur-Gonzales J, Alcántara-Bocanegra F, Pizarro M.A, Guerra R.C, Mori-Pinedo L, and Chu-Koo F. Paco Piaractus brachypomus y gamitana Colossoma macropomum criados en policultivo con el bujurqui-tucanré, Chaetobranchus semifasciatus (Cichlidae) Folia Amazón. 2009;18(1):97–104. [Google Scholar]
- Tang H.G, Wu T.X, Zhao Z.I, and Pan X.D. Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.) J. Zhejiang Univ. Sci. 2008;9(9):684–690. doi: 10.1631/jzus.B0820088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco C, Comesaña S, Conde-Sieira M, Míguez J.M, and Soengas J.L. Effects of CCK-8 and GLP-1 on fatty acid sensing and food intake regulation in trout. J. Mol. Endocrinol. 2019;62(3):101–116. doi: 10.1530/JME-18-0212. [DOI] [PubMed] [Google Scholar]
- Yi Y, Zhang Z, Zhao F, Liu H, Yu L, Zha J, and Wang G. Probiotic potential of Bacillus velezensis JW: antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish Shellfish Immunol. 2018;78:322–330. doi: 10.1016/j.fsi.2018.04.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


