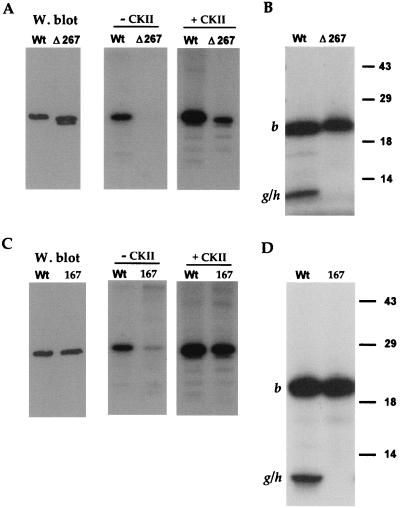
FIG. 3.
VP22 possesses a C-terminal phosphorylation site. (A) Extracts were made from COS-1 cells transfected with plasmids expressing either WT or Δ267 protein. These extracts were analyzed by Western blotting using antibody AGV30 (W. blot) and by immunoprecipitation with AGV30 followed by in vitro phosphorylation in the absence (− CKII) or presence (+ CKII) of CKII. The doublet in lane Δ267 of the Western blot represents full-length Δ267 (upper band) and a slightly smaller breakdown product (lower band). (B) The samples from the in vitro CKII phosphorylation in panel A were transferred to nitrocellulose and subjected to cleavage with endolysine C. The resulting peptides were analyzed by SDS-PAGE on a 15% acrylamide gel. The positions of cleavage peptide b and the partial cleavage product encompassing cleavage peptides g and h (g/h) are indicated. (C) Extracts were made from COS-1 cells transfected with plasmids expressing either WT or pGE167 (167) protein. These extracts were analyzed by Western blotting using antibody AGV30 (W. blot) and by immunoprecipitation with AGV30 followed by in vitro phosphorylation in the absence (− CKII) or presence (+ CKII) of CKII. (D) The samples from the in vitro CKII phosphorylation in panel A were transferred to nitrocellulose and subjected to cleavage with endolysine C. The resulting peptides were analyzed by SDS-PAGE on a 15% acrylamide gel. The positions of cleavage peptide b and the partial cleavage product encompassing cleavage peptides g and h (g/h) are indicated.