Abstract
In this hospital-based cross-sectional analytic study, we retrospectively reviewed clinical data of patients with acute ischemic stroke (AIS) between January 2017 and April 2023. Atrial cardiopathy was defined as any presence of the following: left atrial diameter ≥ 52 mm (males) or ≥ 47 mm (females), elevated P-wave terminal force in V1 > 5000 μV ms, or serum N terminal pro B type natriuretic peptide > 250 pg/ml. Initial stroke severity was defined by the National Institutes of Health Stroke Scale (NIHSS; moderate-to-severe, ≥ 5; and severe, ≥ 15). Univariate and multivariate binary logistic regression analyses were performed to assess the association between atrial cardiopathy and stroke severity. Among 662 AIS patients (mean age 70 years [interquartile range 61–78], 31.3% women), 303 (45.8%) had atrial cardiopathy. Multivariable logistic regression analysis showed that the presence of atrial cardiopathy was significantly associated with a higher odd of moderate-to-severe stroke (adjusted odds ratio [OR] 2.16, 95% confidence interval [CI] 1.46–3.20, p < 0.001) and severe stroke (adjusted OR 4.89, 95%CI 2.45–9.76, p < 0.001). This association remained significant in a sensitivity analysis excluding those with atrial fibrillation or coronary artery disease. Findings of the current study revealed that the association of atrial cardiopathy was with initial stroke severity independent of atrial fibrillation and was even confirmed in patients without atrial fibrillation; future studies to explore improved stroke prevention strategies for patients with atrial cardiopathy are needed.
Keywords: Acute ischemic stroke, Atrial cardiopathy, Stroke severity, National institutes of health stroke scale
Subject terms: Diseases of the nervous system, Cardiology, Neurology
Introduction
Atrial cardiopathy is characterized by changes in atrial structure or function, such as atrial fibrosis, impaired cardiac cell function, and atrial enlargement, which may precede and promote atrial fibrillation1–3. Although currently there is no golden standard to define atrial cardiopathy, established biomarkers of atrial cardiopathy include increased P-wave terminal force in lead V1 (PTFV1) on electrocardiogram, left atrial enlargement (LAE) on echocardiogram, and elevated serum N-terminal pro-brain natriuretic peptide (NT-proBNP)4. In stroke free individuals, increased PTFV1 was associated with incident stroke risk5,6 in the absence of clinically apparent atrial fibrillation7. Observational evidence showed that LAE on echocardiogram was independently associated with an increased risk of ischemic stroke in the absence of atrial fibrillation8–10. Moreover, increased serum level of NT-proBNP, a biomarker of ventricular and atrial dysfunction, is also associated with ischemic stroke11,12.
Previous studies have shown that atrial cardiopathy might be useful to assess the probability of cardioembolism in patients with cryptogenic stroke13. However, whether atrial cardiopathy relates to stroke severity has been scarcely reported. The influence of initial stroke severity on stroke recovery has been increasingly recognized. For example, moderate to severe stroke has been shown to be associated with mortality, increasing the likelihood of in-hospital mortality by more than nine-fold14,15. Several previous studies assessed the association between atrial cardiopathy and stroke severity in acute ischemic stroke (AIS) patients, yielding inconsistent findings. A small cohort of the Stepwise screening for silent atrial fibrillation after stroke (SAFAS) study showed a significant difference in the National Institutes of Health Stroke Scale (NIHSS) score in patients with and without atrial cardiopathy, suggesting a potential link between atrial cardiopathy and stroke severity16. However, a small prospective cohort study showed that the proportion of a higher NIHSS score (NIHSS of 9 or above) was similar in participants with and without atrial cardiopathy (10 [14%] vs. 6 [13%], p = 0.91)17. To our knowledge, the association between atrial cardiopathy and stroke severity in AIS patients remains not fully understood. We therefore aimed to investigate whether the presence of atrial cardiopathy is associated with stroke severity in AIS population.
Methods
Participants
In this hospital-based cross-sectional analytic study, we retrospectively reviewed clinical data of adult patients with AIS between January 2017 and April 2023 in a university teaching hospital. The exclusion criteria were as follows: (1) Recorded rheumatic heart valve disease or artificial heart valve; (2) Without adequate electrocardiogram parameters to assess PTFV1; (3) Without transthoracic echocardiogram assessment of left atrial diameter; (4) Without NT-proBNP data. We collected the demographic and clinical characteristics including age, sex, body mass index, alcohol and cigarette consumption, history of hypertension, hyperlipidemia, diabetes, atrial fibrillation, and coronary heart disease from patients’ medical notes. Atrial fibrillation included paroxysmal, persistent, and permanent atrial fibrillation, which was either previously known or diagnosed during index hospitalization18. Etiologic subtypes of ischemic stroke were classified according to the TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria19. Laboratory findings (fasting blood glucose, triglyceride, total cholesterol, low-density lipoprotein, and high-density lipoprotein) were collected from a digital medical database. The study protocol was reviewed and approved by the Fujian Medical University Union Hospital Ethics Committees (2023KY030). Informed consent was waived, due to the nature of the retrospective study with routine anonymous and de-identified information. This study was conducted in accordance with the Declaration of Helsinki and relevant guidelines and regulations. The datasets of the current study are available from the corresponding author on reasonable request.
Atrial cardiopathy assessment
Biomarkers of atrial cardiopathy were routinely assessed within 48 h after admission. PTFV1 in electrocardiogram was calculated by multiplying the duration (ms) of the terminal negative component of P-wave by its amplitude (µV) in lead V1 recorded by manual calculation20. Left atrium size was calculated as the antero-posterior maximal diameter of the left atrium in systole on B-mode transthoracic echocardiogram. Severe left atrial enlargement was defined as left atrial diameter ≥ 52 mm (males) or ≥ 47 mm (females)21. NT-proBNP was measured by an automated electrochemiluminescence immunoassay (Elecsys proBNP II assay). The coefficient of variation for the NT-proBNP assay was 2–5% during the testing period, and the analytical measurement range for NT-proBNP was 5–35,000 pg/ml. In the present study, atrial cardiopathy was defined as any of the following: severe LAE, PTFV1 > 5000 μV ms, or increased serum levels of NT-proBNP > 250 pg/ml22.
Stroke severity assessment
Initial stroke severity was assessed using the NIHSS on admission. Moderate-to-severe stroke was defined as a NIHSS scored of 5 or higher, and severe stroke as a NIHSS score of 15 or higher23.
Statistical analysis
Continuous variables were presented as means (standard deviation) if normally distributed, or medians (interquartile range, IQR) if not normally distributed. Categorical variables were expressed as frequencies with proportions. Between group differences were compared using the t-test, the Mann–Whitney test, the Chi-square test, or the Fisher’s exact test as appropriate. Logistic regression models were used to calculate the unadjusted and adjusted odds ratios (ORs) of outcomes with atrial cardiopathy. Two models were used: Model 1, Age- and sex- adjusted; and Model 2, adjusted for variables with a p < 0.1 in the univariate analysis for outcomes. We conducted several subgroup analyses of outcomes stratified by age group (< 65 vs. ≥ 65 years), sex (male vs. female), smoking status (current smoker vs. not), alcohol consumption (regular vs. not), hypertension (yes vs. no), dyslipidemia (yes vs. no), coronary artery disease (yes vs. no), and atrial fibrillation (yes vs. no), with the interaction terms as covariates. We conducted a sensitivity analysis by including patients without atrial fibrillation or coronary artery disease. We additionally assessed the linear relationship between atrial cardiopathy and continuous NIHSS score. A p-value < 0.05 was considered statistically significant. All statistical analyses were done using SPSS version 26.0 (IBM Corp., Armonk, NY, USA).
Ethical approval
This study protocol was reviewed and approved by Fujian Medical University Union Hospital Ethics Committees (2023KY030). Written informed consent was waived by the Fujian Medical University Union Hospital Ethics Committees due to the nature of our retrospective study based on routine clinical data.
Results
Baseline characteristics
A total of 662 AIS patients (mean age 70 years [IQR 61–78], 31.3% women) who met the inclusion criteria were finally analyzed (Fig. S1). A total of 303 (45.8%) patients had atrial cardiopathy. Table 1 shows the baseline characteristics between participants in the acute ischemic stroke with and without atrial cardiopathy. Patients with atrial cardiopathy were older (74 years [IQR 65–81] vs. 66 [IQR 58–73], p < 0.001), more likely to be female (35.3% vs. 27.9%, p = 0.039), and less likely to be current smoker (23.1% vs. 34.3%, p = 0.002) and regular alcohol user (9.0% vs. 17.0%, p = 0.008). The prevalence of hypertension (74.3% vs. 60.4%, p < 0.001), atrial fibrillation (48.2% vs. 5.0%, p < 0.001), coronary artery disease (22.8% vs. 8.1%, p < 0.001), pre-stroke anticoagulation (7.6% vs. 0.6%, p < 0.001), and the level of high-density lipoprotein (1.14 ± 0.31 vs. 1.07 ± 0.25 mmol/L, p = 0.004) were higher, while the proportion of dyslipidemia and serum levels of triglyceride were lower among patients with atrial cardiopathy compared to those without atrial cardiopathy. Stroke subtypes in patients with and without atrial cardiopathy were significantly different (p < 0.001).
Table 1.
Baseline characteristics between patients with and without atrial cardiopathy.
| Characteristics | Total (n = 662) | Without atrial cardiopathy (n = 359) | With atrial cardiopathy (n = 303) | p |
|---|---|---|---|---|
| Age, y, median (IQR) | 70 (61–78) | 66 (58–73) | 74 (65–81) | < 0.001 |
| Sex, female, n (%) | 207 (31.3) | 100 (27.9) | 107 (35.3) | 0.039 |
| NIHSS score, median (IQR) | 3 (1–8) | 3 (1–5) | 5 (2–12) | < 0.001 |
| BMI, kg/m2, mean ± SD | 23.7 ± 3.5 | 23.7 ± 3.2 | 23.7 ± 3.9 | 0.218 |
| Current smoker, n (%) | 193 (29.2) | 123 (34.3) | 70 (23.1) | 0.002 |
| Regular alcohol user, n (%) | 91 (13.7 | 61 (17.0) | 30 (9.0) | 0.008 |
| Hypertension, n (%) | 442 (66.8) | 217 (60.4) | 225 (74.3) | < 0.001 |
| Diabetes, n (%) | 218 (32.9) | 121 (33.7) | 97 (32.0) | 0.645 |
| Atrial fibrillation, n (%) | 164 (24.8) | 18 (5.0) | 146 (48.2) | < 0.001 |
| Dyslipidemia, n (%) | 166 (25.1) | 104 (29.0) | 62 (20.5) | 0.012 |
| Coronary artery disease, n (%) | 98 (14.8) | 29 (8.1) | 69 (22.8) | < 0.001 |
| Pre-stroke anticoagulation, n (%) | 25 (3.8) | 2 (0.6) | 23 (7.6) | < 0.001 |
| Pre-stroke antiplatelet drug, n (%) | 24 (3.6) | 10 (2.8) | 14 (4.6) | 0.208 |
| Wake-up stroke, n (%) | 89 (13.4) | 49 (13.6) | 40 (13.2) | 0.866 |
| TOAST subtypes, n (%) | < 0.001 | |||
| Large artery atherosclerosis | 436 (65.9) | 284 (79.1) | 152 (50.2) | |
| Cardio-embolism | 177 (26.7) | 37 (10.3) | 140 (46.2) | |
| Small vessel occlusion | 22 (3.3) | 16 (4.5) | 6 (2.0) | |
| Undetermined | 11 (1.7) | 8 (2.2) | 3 (1.0) | |
| Others | 16 (2.4) | 14 (3.9) | 2 (0.7) | |
| FBG, mmol/L, median (IQR) | 6.09 (5.14–7.74) | 5.95 (5.03–7.74) | 6.35 (5.32–7.83) | 0.051 |
| Triglyceride, mmol/L, median (IQR) | 1.21 (0.94–1.64) | 1.31 (1.01–1.78) | 1.09 (0.88–1.46) | < 0.001 |
| Total cholesterol, mmol/L, mean ± SD | 4.43 ± 1.07 | 4.45 ± 0.96 | 4.39 ± 0.96 | 0.251 |
| LDL, mmol/L, mean ± SD | 2.95 ± 0.99 | 2.97 ± 0.89 | 2.92 ± 1.10 | 0.227 |
| HDL, mmol/L, mean ± SD | 1.10 ± 0.28 | 1.07 ± 0.25 | 1.14 ± 0.31 | 0.004 |
IQR interquartile range, NIHSS National Institutes of Health Stroke Scale, SD standard deviation, BMI body mass index, TOAST Trial of Org 10172 in Acute Stroke Treatment, FBG fasting blood glucose, LDL low-density lipoprotein, HDL high-density lipoprotein.
Atrial cardiopathy and stroke severity
Patients with atrial cardiopathy had a higher NIHSS score (5 [IQR 2–12] vs. 3 [IQR 1–5], p < 0.001). A total of 261 (39.4%) patients had a moderate-to-severe stroke, and 70 (10.6%) had a severe stroke. Table 2 summarizes the differences in characteristics in patients with and without moderate-to-severe stroke. Compared to those with mild stroke (NIHSS < 5), those with moderate-to-severe stroke (NIHSS ≥ 5) were more likely to be female (36.8% vs. 27.7%, p = 0.014) and had a higher proportion of atrial fibrillation (29.9% vs. 21.4%, p = 0.014), coronary artery disease (18.8% vs. 12.2%, p = 0.020), a higher serum level of fasting blood glucose (6.53 [5.38–8.09] vs. 5.83 [5.05–7.56] mmol/L, p < 0.001), and a lower triglyceride level (1.16 [0.92–1.48] vs. 1.25 [0.96–1.75] mmol/L, p = 0.016). Stroke subtypes in patients with and without moderate-to-severe stroke were significantly different (p < 0.001). Of the individual atrial cardiopathy biomarkers, patients with moderate-to-severe stroke were more likely to have an NT-proBNP > 250 pg/mL (55.9% vs. 35.4%, p < 0.001). Similar findings were detected in patients with severe stroke (NIHSS ≥ 15) compared to those with non-severe stroke (NIHSS < 15) (Table 3).
Table 2.
Baseline characteristics in patients with and without moderate-to-severe stroke.
| Characteristics | Without moderate-to-severe stroke (n = 401) | With moderate-to-severe stroke (n = 261) | p |
|---|---|---|---|
| Age, y, median (IQR) | 69 (61–77) | 71 (61–79) | 0.198 |
| Sex, female, n (%) | 111 (27.7) | 96 (36.8) | 0.014 |
| BMI, kg/m2, mean ± SD | 23.8 ± 3.5 | 23.6 ± 3.7 | 0.204 |
| Current smoker, n (%) | 121 (30.2) | 72 (27.6) | 0.474 |
| Regular alcohol user, n (%) | 58 (14.5) | 33 (12.6) | 0.506 |
| Hypertension, n (%) | 269 (67.1) | 173 (66.3) | 0.831 |
| Diabetes, n (%) | 133 (33.2) | 85 (32.6) | 0.872 |
| Atrial fibrillation, n (%) | 86 (21.4) | 78 (29.9) | 0.014 |
| Dyslipidemia, n (%) | 110 (27.4) | 56 (21.5) | 0.083 |
| Coronary artery disease, n (%) | 49 (12.2) | 49 (18.8) | 0.020 |
| Pre-stroke anticoagulation, n (%) | 18 (4.5) | 7 (2.7) | 0.233 |
| Pre-stroke antiplatelet drug, n (%) | 14 (3.5) | 10 (3.8) | 0.819 |
| Wake-up stroke, n (%) | 46 (11.5) | 43 (16.5) | 0.065 |
| TOAST subtypes, n (%) | 0.009 | ||
| Large artery atherosclerosis | 264 (65.8) | 172 (65.9) | |
| Cardio-embolism | 99 (24.7) | 78 (29.9) | |
| Small vessel occlusion | 21 (5.2) | 1 (0.4) | |
| Undetermined | 6 (1.5) | 5 (1.9) | |
| Others | 11 (2.7) | 5 (1.9) | |
| FBG, mmol/L, median (IQR) | 5.83 (5.05–7.56) | 6.53 (5.38–8.09) | < 0.001 |
| Triglyceride, mmol/L, median (IQR) | 1.25 (0.96–1.75) | 1.16 (0.92–1.48) | 0.016 |
| Total cholesterol, mmol/L, mean ± SD | 4.39 ± 1.02 | 4.48 ± 1.14 | 0.388 |
| LDL, mmol/L, mean ± SD | 2.91 ± 0.93 | 3.00 ± 1.07 | 0.431 |
| HDL, mmol/L, mean ± SD | 1.09 ± 0.28 | 1.11 ± 0.29 | 0.378 |
| Atrial Cardiopathy, n (%) | 151 (37.7) | 152 (58.2) | < 0.001 |
| PTFV1 > 5000 μV·ms, n (%) | 19 (4.7) | 10 (3.8) | 0.577 |
| Severe LAE, n (%) | 22 (5.5) | 12 (4.6) | 0.613 |
| NT-proBNP > 250 pg/ml, n (%) | 142 (35.4) | 146 (55.9) | < 0.001 |
IQR interquartile range, BMI body mass index, SD standard deviation, TOAST Trial of Org 10172 in Acute Stroke Treatment, FBG fasting blood glucose, LDL low-density lipoprotein, HDL high-density lipoprotein, PTFV1 P-wave terminal force in lead V1, LAE left atrial enlargement, NT-proBNP N-terminal pro-brain natriuretic peptide.
Table 3.
Baseline characteristics in patients with and without severe stroke.
| Characteristics | Without severe stroke (n = 592) | With severe stroke (n = 70) | p |
|---|---|---|---|
| Age, y, median (IQR) | 69 (61–77) | 73 (62–83) | 0.033 |
| Sex, female, n (%) | 181 (30.6) | 26 (37.1) | 0.262 |
| BMI, kg/m2, mean ± SD | 23.8 ± 3.5 | 23.5 ± 3.6 | 0.347 |
| Current smoker, n (%) | 178 (30.1) | 15 (21.4) | 0.133 |
| Regular alcohol user, n (%) | 82 (13.9) | 9 (12.9) | 0.819 |
| Hypertension, n (%) | 391 (66.0) | 51 (72.9) | 0.253 |
| Diabetes, n (%) | 201 (34.0) | 17 (24.3) | 0.104 |
| Atrial fibrillation, n (%) | 134 (22.6) | 30 (42.9) | < 0.001 |
| Dyslipidemia, n (%) | 156 (26.4) | 10 (14.3) | 0.028 |
| Coronary artery disease, n (%) | 81 (13.7) | 17 (24.3) | 0.018 |
| Pre-stroke anticoagulation, n (%) | 23 (3.9) | 2 (2.9) | 0.670 |
| Pre-stroke antiplatelet drug, n (%) | 20 (3.4) | 4 (5.7) | 0.323 |
| Wake-up stroke, n (%) | 80 (13.5) | 9 (12.9) | 0.879 |
| TOAST subtypes, n (%) | 0.021 | ||
| Large artery atherosclerosis | 397 (67.1) | 39 (55.7) | |
| Cardio-embolism | 148 (25.0) | 29 (41.4) | |
| Small vessel occlusion | 22 (3.7) | 0 (0.0) | |
| Undetermined | 11 (1.9) | 0 (0.0) | |
| Others | 14 (2.4) | 2 (2.9) | |
| FBG, mmol/L, median (IQR) | 6.03 (5.07–7.74) | 6.86 (5.86–8.09) | 0.001 |
| Triglyceride, mmol/L, median (IQR) | 1.23 (0.95–1.69) | 1.09 (0.86–1.40) | 0.004 |
| Total cholesterol, mmol/L, mean ± SD | 4.46 ± 1.13 | 4.11 ± 1.10 | 0.005 |
| LDL, mmol/L, mean ± SD | 2.98 ± 0.98 | 2.67 ± 0.99 | 0.005 |
| HDL, mmol/L, mean ± SD | 1.10 ± 0.27 | 1.12 ± 0.32 | 0.654 |
| Atrial Cardiopathy, n (%) | 247 (41.7) | 56 (80.0) | < 0.001 |
| PTFV1 > 5000 μV ms, n (%) | 25 (4.2) | 4 (5.7) | 0.564 |
| Severe LAE, n (%) | 30 (5.1) | 4 (5.7) | 0.817 |
| NT-proBNP > 250 pg/ml, n (%) | 234 (39.5) | 54 (77.1) | < 0.001 |
IQR interquartile range, SD standard deviation, BMI body mass index, TOAST Trial of Org 10,172 in Acute Stroke Treatment, FBG fasting blood glucose, LDL low-density lipoprotein, HDL high-density lipoprotein, PTFV1 P-wave terminal force in lead V1, LAE left atrial enlargement, NT-proBNP N-terminal pro-brain natriuretic peptide.
Table 4 shows the association between atrial cardiopathy and stroke severity. Patients with atrial cardiopathy were at a higher odd of moderate-to-severe stoke (unadjusted OR 2.31, 95%CI 1.68–3.17, p < 0.001) and severe stroke (unadjusted OR 5.58, 95%CI 3.04–10.26, p = 0.001). The associations remained after adjustment for age and sex. After further adjustment for those variables with a p < 0.1 in the univariable analysis, atrial cardiopathy remained significantly associated with a higher odd of moderate-to-severe stoke (adjusted OR 2.16, 95%CI 1.46–3.20, p < 0.001) and severe stroke (adjusted OR 4.89, 95%CI 2.45–9.76, p < 0.001). This association remained significant when considering the NIHSS score as a continuous variable (unadjusted Beta 0.292, adjusted Beta 0.280, p < 0.001, respectively; Table 4).
Table 4.
Association between atrial cardiopathy and stroke severity.
| Moderate-to-severe stroke | Severe stroke | Continuous NIHSS | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | Beta | p | |
| Univariate | 2.31 (1.68–3.17) | < 0.001 | 5.58 (3.04–10.26) | 0.001 | 0.292 | < 0.001 |
| Model 1 | 2.33 (1.67–3.26) | < 0.001 | 5.40 (2.88–10.11) | 0.001 | 0.287 | < 0.001 |
| Model 2 | 2.16 (1.46–3.20) | < 0.001 | 4.89 (2.45–9.76) | < 0.001 | 0.280 | < 0.001 |
| Sensitivity analyses | ||||||
| Univariate | 2.07 (1.35–3.17) | 0.001 | 5.15 (2.45–10.83) | < 0.001 | 0.259 | < 0.001 |
| Model 1 | 2.37 (1.51–3.73) | < 0.001 | 5.07 (2.34–11.02) | < 0.001 | 0.274 | < 0.001 |
| Model 2 | 2.23 (1.40–3.55) | < 0.001 | 4.76 (2.13–10.61) | < 0.001 | 0.266 | < 0.001 |
Model 1: Age and sex adjusted;
Model 2: Adjusted for variables with a p < 0.1 in the univariate analysis for outcomes; (age, sex, dyslipidemia, atrial fibrillation, coronary artery disease, wake-up stroke, TOAST, FBG, triglyceride, total cholesterol, LDL).
Sensitivity analysis: excluding patients with atrial fibrillation or coronary artery disease. Two models were used:
Model 1: Age and sex adjusted;
Model 2: Adjusted for age, sex, dyslipidemia, wake-up stroke, TOAST, FBG, triglyceride, total cholesterol, LDL.
NIHSS National Institutes of Health Stroke Scale, OR odds ratio, CI confidence interval, TOAST Trial of Org 10172 in Acute Stroke Treatment, FBG fasting blood glucose, LDL low-density lipoprotein.
Subgroup analysis and sensitivity analysis
We detected no significant interactions between atrial cardiopathy and variables including age (< 65 vs. ≥ 65 years), sex (male vs. female), smoking status (current smoker vs. not), alcohol consumption (regular vs. not), hypertension (yes vs. no), dyslipidemia (yes vs. no), coronary artery disease (yes vs. no), and atrial fibrillation (yes vs. no) for stroke severity (Fig. 1a,b). We observed a significant association of atrial cardiopathy with moderate to severe stroke (OR 2.31, 95%CI 1.57–3.40, p < 0.001) and severe stroke (OR 4.64, 95%CI 2.35–9.16, p < 0.001) in patients without atrial fibrillation. In the presence of atrial fibrillation, there was trend for a higher odd of moderate to severe stroke with atrial cardiopathy, although this did not reach statistical significance (OR 2.60, 95%CI 0.88–7.67, p = 0.083; Fig. 1a).
Figure 1.
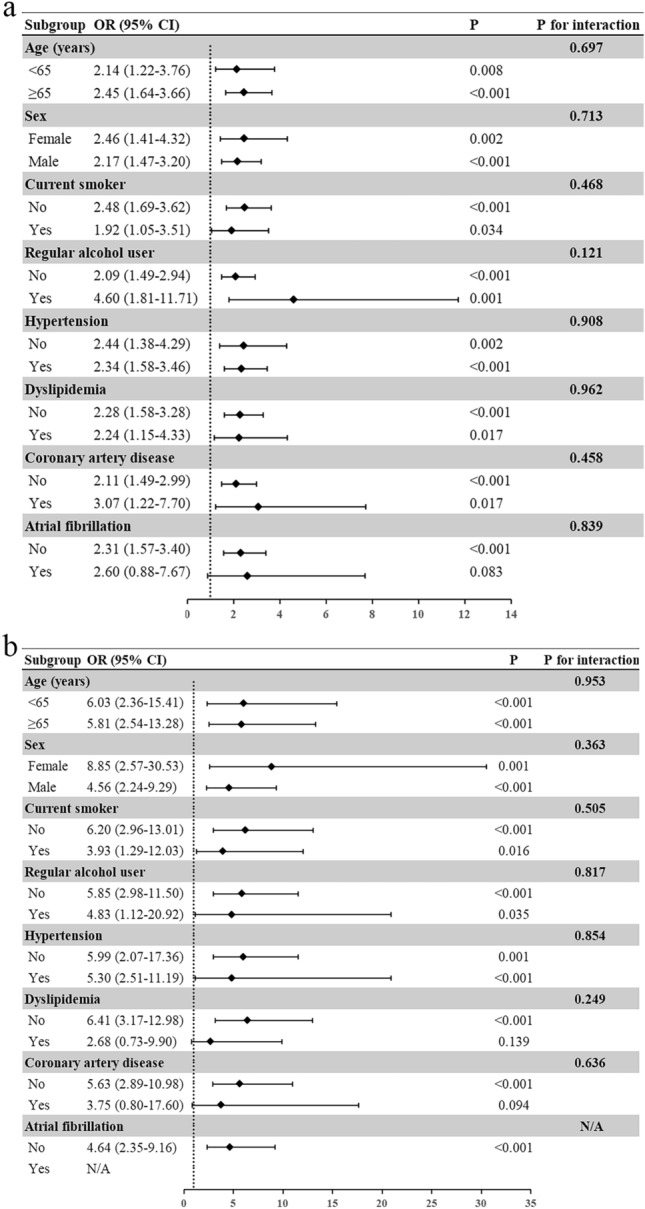
The association between atrial cardiopathy and stroke severity in different subgroups. (a) The association between atrial cardiopathy and moderate-to-severe stroke (NIHSS ≥ 5) in different subgroups. (b) The association between atrial cardiopathy and severe stroke (NIHSS ≥ 15) in different subgroups. Abbreviations: OR, odds ratio; CI, confidence interval.
A sensitivity analysis after excluding patients with atrial fibrillation or coronary artery disease showed that atrial cardiopathy remained associated with moderate-to-severe stroke as well as severe stroke (Table 4).
Discussion
The current study showed that atrial cardiopathy was associated with initial stroke severity, suggesting that atrial cardiopathy may serve as a clinical indicator of stroke severity in AIS population. Our findings underscore the importance of increasing awareness and attention to this relationship.
Atrial cardiopathy is independently associated with incident ischemic stroke. A recent meta-analysis of observational cohort data showed that the biomarkers of atrial cardiopathy were significantly associated with the risk of ischemic stroke (increased PTFV1, hazards ratio [HR] 1.29, 95%CI 1.06–1.57; LAE, HR 1.39, 95% CI 1.06–1.82; and increased NT-proBNP, HR 2.37, 95%CI 1.61–3.50)4. In a retrospective longitudinal cohort of 32,454 community-dwelling old participants, atrial cardiopathy was independently associated with an increased risk of ischemic stroke in the absence of atrial fibrillation8. Data from the Third China National Stroke Registry (CNSR-III) also showed an association of poor prognosis with atrial cardiopathy in patients with ischemic stroke, even when patients with atrial fibrillation were excluded24. The Cardiovascular Health Study of community-dwelling elderly adults who were free of stroke and atrial fibrillation at baseline showed that atrial cardiopathy was independently associated with incident ischemic stroke25. Our results build on the above-mentioned studies by showing an independent association between atrial cardiopathy and index stroke severity, suggesting the potential prognostic role of atrial cardiopathy in AIS population. In line with our findings, an observational study of 1271 AIS patients showed that LAE was associated with more severe initial neurologic deficits of embolic subtypes (cardioembolic and cryptogenic stroke)26. However, the small number of patients with cryptogenic stroke (n = 11) in our cohort does not allow us to assess the relationship between stroke severity and atrial cardiopathy in this subgroup. On the contrary, a retrospective analysis of the Henry Ford Health System did not detect the association between atrial cardiopathy and stroke risk27. Possible explanations for the disparities in the above-mentioned studies may include the different study population and different definition for atrial cardiopathy.
The association between atrial cardiopathy and stroke severity might be partly explained by the relationship between atrial cardiopathy and atrial fibrillation, a well-established condition that increases the risk of severe stroke28. This hypothesis is supported by our data showing that patients with atrial cardiopathy were more likely to have atrial fibrillation than those without atrial cardiopathy (48.2% vs. 5.0%, p < 0.001). Interestingly, our subgroup analysis showed only a trend for a higher odd of moderate to severe stroke with atrial cardiopathy in those with atrial fibrillation, while this association was quite significant in patients without atrial fibrillation. This is probably attributable to the fact that most patients with atrial fibrillation have atrial cardiopathy as defined in our participants, independent of the severity of their stroke. In line with our findings, a small observational study showed that LAE, a biomarker for atrial cardiopathy, was associated with severe ischemic stroke in men with nonvalvular atrial fibrillation, suggesting that atrial cardiopathy may play a crucial role for thrombogenesis in atrial fibrillation29. Moreover, several recent studies have shown that atrial cardiopathy may be a cause of cardioembolic stroke even in the absence of atrial fibrillation5,6,30. In agreement of the aforementioned studies, our data also showed that patients with atrial cardiopathy were more likely to have a cardioembolic stroke (46.2% vs. 10.3%). Our findings need to be validated in future large prospective studies.
Our study has several limitations. First, our study is a single-center retrospective observational study with inevitable selection bias. Second, our study lacked the data of LA volume, which is a more reliable estimator of LA size31. However, LA diameter is more readily available and more widely used in clinical practice. Third, we only analyzed Chinese stroke patients; our findings may therefore not be generalizable to other populations. Moreover, our findings should be interpreted with caution due to unmeasured potential confounding effects of volume expansion for stroke management and stress-induced cardiomyopathy. Finally, we only assess the association of atrial cardiopathy with stroke severity cross-sectionally, studies with a long-term follow-up will better evaluate the relationship between atrial cardiopathy and long-term prognosis of acute ischemic stroke.
Conclusion
This study showed that atrial cardiopathy was significantly associated with stroke severity, suggesting that atrial cardiopathy may help in predicting stroke prognosis. Future studies are needed to explore improved stroke prevention strategies for patients with atrial cardiopathy.
Supplementary Information
Abbreviations
- AIS
Acute ischemic stroke
- NIHSS
National Institutes of Health Stroke Scale
- OR
Odds ratio
- CI
Confidence interval
- PTFV1
P-wave terminal force in lead V1
- LAE
Left atrial enlargement
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- SAFAS
Stepwise screening for silent atrial fibrillation after stroke
- IQR
Interquartile range
- HR
Hazards ratio
- CNSR-III
Third China National Stroke Registry
Author contributions
Concept and design: Y.Z., H.Lei., and H.D.; Acquisition, analysis, or interpretation of data: Y.Z., H.Lei., X.W., H.Lin. and H.D.; Drafting of the manuscript: Y.Z., H.Lei., and H.D.; Critical revision of the manuscript for important intellectual content: S.F., Q.Y., N.L.; H.D. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was funded by the Fujian Provincial Clinical Research Center for Brain Diseases (2021FJSLCYX01). The funder had no role in the design, data collection, data analysis, and reporting of this study.
Data availability
Data are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yidan Zhang and Hanhan Lei.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-61304-y.
References
- 1.Kamel, H., Okin, P. M., Longstreth, W. T., Elkind, M. S. V. & Soliman, E. Z. Atrial cardiopathy: A broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiol.11, 323–331. 10.2217/fca.15.22 (2015). 10.2217/fca.15.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calenda, B. W., Fuster, V., Halperin, J. L. & Granger, C. B. Stroke risk assessment in atrial fibrillation: Risk factors and markers of atrial myopathy. Nat. Rev. Cardiol.13, 549–559. 10.1038/nrcardio.2016.106 (2016). 10.1038/nrcardio.2016.106 [DOI] [PubMed] [Google Scholar]
- 3.Goette, A. et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm14, e3–e40. 10.1016/j.hrthm.2016.05.028 (2017). 10.1016/j.hrthm.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo, J. et al. Atrial cardiomyopathy and incident ischemic stroke risk: A systematic review and meta-analysis. J. Neurol.270, 3391–3401. 10.1007/s00415-023-11693-3 (2023). 10.1007/s00415-023-11693-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamel, H. et al. P-wave morphology and the risk of incident ischemic stroke in the multi-ethnic study of atherosclerosis. Stroke45, 2786–2788. 10.1161/STROKEAHA.114.006364 (2014). 10.1161/STROKEAHA.114.006364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamel, H. et al. Electrocardiographic left atrial abnormality and risk of stroke: Northern Manhattan study. Stroke46, 3208–3212. 10.1161/STROKEAHA.115.009989 (2015). 10.1161/STROKEAHA.115.009989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He, J. et al. P-wave indices and risk of ischemic stroke: A systematic review and meta-analysis. Stroke48, 2066–2072. 10.1161/STROKEAHA.117.017293 (2017). 10.1161/STROKEAHA.117.017293 [DOI] [PubMed] [Google Scholar]
- 8.Edwards, J. D., Healey, J. S., Fang, J., Yip, K. & Gladstone, D. J. Atrial cardiopathy in the absence of atrial fibrillation increases risk of ischemic stroke, incident atrial fibrillation, and mortality and improves stroke risk prediction. J. Am. Heart Assoc.9, e013227. 10.1161/JAHA.119.013227 (2020). 10.1161/JAHA.119.013227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes, M. E. et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin. Proc.79, 1008–1014 (2004). 10.4065/79.8.1008 [DOI] [PubMed] [Google Scholar]
- 10.Benjamin, E. J., D’Agostino, R. B., Belanger, A. J., Wolf, P. A. & Levy, D. Left atrial size and the risk of stroke and death. The Framingham heart study. Circulation92, 835–841 (1995). 10.1161/01.CIR.92.4.835 [DOI] [PubMed] [Google Scholar]
- 11.Folsom, A. R. et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: The atherosclerosis risk in communities study. Stroke44, 961–967. 10.1161/STROKEAHA.111.000173 (2013). 10.1161/STROKEAHA.111.000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cushman, M. et al. N-terminal pro-B-type natriuretic peptide and stroke risk: The reasons for geographic and racial differences in stroke cohort. Stroke45, 1646–1650. 10.1161/STROKEAHA.114.004712 (2014). 10.1161/STROKEAHA.114.004712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalini, S. et al. Atrial cardiopathy in patients with embolic strokes of unknown source and other stroke etiologies. Neurology92, e288–e294. 10.1212/WNL.0000000000006748 (2019). 10.1212/WNL.0000000000006748 [DOI] [PubMed] [Google Scholar]
- 14.Messé, S. R. et al. Stroke after aortic valve surgery: results from a prospective cohort. Circulation129, 2253–2261. 10.1161/CIRCULATIONAHA.113.005084 (2014). 10.1161/CIRCULATIONAHA.113.005084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long, X. et al. Mortality, recurrence, and dependency rates are higher after acute ischemic stroke in elderly patients with diabetes compared to younger patients. Front. Aging Neurosci.8, 142. 10.3389/fnagi.2016.00142 (2016). 10.3389/fnagi.2016.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Didier, R. et al. Distribution of atrial cardiomyopathy markers and association with atrial fibrillation detected after ischaemic stroke in the SAFAS study. Stroke Vasc. Neurol.10.1136/svn-2023-002447 (2023). 10.1136/svn-2023-002447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao, D. X., Gootee, E. & Johansen, M. C. Atrial cardiopathy is associated with cerebral microbleeds in ischemic stroke patients. Front. Neurol.13, 982926. 10.3389/fneur.2022.982926 (2022). 10.3389/fneur.2022.982926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldgren, J. et al. Early versus delayed non-vitamin K antagonist oral anticoagulant therapy after acute ischemic stroke in atrial fibrillation (TIMING): A registry-based randomized controlled noninferiority study. Circulation146, 1056–1066. 10.1161/CIRCULATIONAHA.122.060666 (2022). 10.1161/CIRCULATIONAHA.122.060666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams, H. P. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke24, 35–41 (1993). 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 20.Soliman, E. Z. et al. Reference ranges of PR duration and P-wave indices in individuals free of cardiovascular disease: The multi-ethnic study of atherosclerosis (MESA). J. Electrocardiol.46, 702–706. 10.1016/j.jelectrocard.2013.05.006 (2013). 10.1016/j.jelectrocard.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hrynkiewicz-Szymanska, A. et al. Association of the CHADS2 and CHA 2DS 2-VASc scores with left atrial enlargement: A prospective cohort study of unselected atrial fibrillation patients. J. Thromb. Thrombolysis40, 240–247. 10.1007/s11239-014-1154-6 (2015). 10.1007/s11239-014-1154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ning, Y., Wei, M., Song, W. & Luo, G. The relationship between atrial cardiopathy biomarkers and prognosis of patients with embolic stroke of undetermined source. Front. Cardiovasc. Med.9, 829361. 10.3389/fcvm.2022.829361 (2022). 10.3389/fcvm.2022.829361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viktorisson, A. et al. Associations of prestroke physical activity with stroke severity and mortality after intracerebral hemorrhage compared with ischemic stroke. Neurology99, e2137–e2148. 10.1212/WNL.0000000000201097 (2022). 10.1212/WNL.0000000000201097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu, Y. et al. Prognostic significance of atrial cardiopathy in patients with acute ischemic stroke. Eur. Stroke J.8, 183–190. 10.1177/23969873221126000 (2023). 10.1177/23969873221126000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamel, H. et al. Atrial cardiopathy and the risk of ischemic stroke in the CHS (cardiovascular health study). Stroke49, 980–986. 10.1161/STROKEAHA.117.020059 (2018). 10.1161/STROKEAHA.117.020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue, J. et al. Left atrial enlargement is associated with stroke severity with cardioembolic and cryptogenic subtypes in a Chinese population. J. Stroke Cerebrovasc. Dis.29, 104767. 10.1016/j.jstrokecerebrovasdis.2020.104767 (2020). 10.1016/j.jstrokecerebrovasdis.2020.104767 [DOI] [PubMed] [Google Scholar]
- 27.Affan, M. et al. Atrial fibrillation, not atrial cardiopathy, is associated with stroke: A single center retrospective study. J. Neurol. Sci.402, 69–73. 10.1016/j.jns.2019.05.012 (2019). 10.1016/j.jns.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 28.Lin, H. J. et al. Stroke severity in atrial fibrillation. The Framingham study. Stroke27, 1760–1764 (1996). 10.1161/01.STR.27.10.1760 [DOI] [PubMed] [Google Scholar]
- 29.Kim, T.-W. et al. Left atrial dilatation is associated with severe ischemic stroke in men with non-valvular atrial fibrillation. J. Neurol. Sci.354, 97–102. 10.1016/j.jns.2015.05.008 (2015). 10.1016/j.jns.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 30.Yaghi, S. et al. Left atrial enlargement and stroke recurrence: The northern Manhattan stroke study. Stroke46, 1488–1493. 10.1161/STROKEAHA.115.008711 (2015). 10.1161/STROKEAHA.115.008711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khankirawatana, B., Khankirawatana, S. & Porter, T. How should left atrial size be reported? Comparative assessment with use of multiple echocardiographic methods. Am. Heart J.147, 369–374 (2004). 10.1016/j.ahj.2003.03.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author on reasonable request.


