Extended Data Fig. 1. CONSORT diagram.
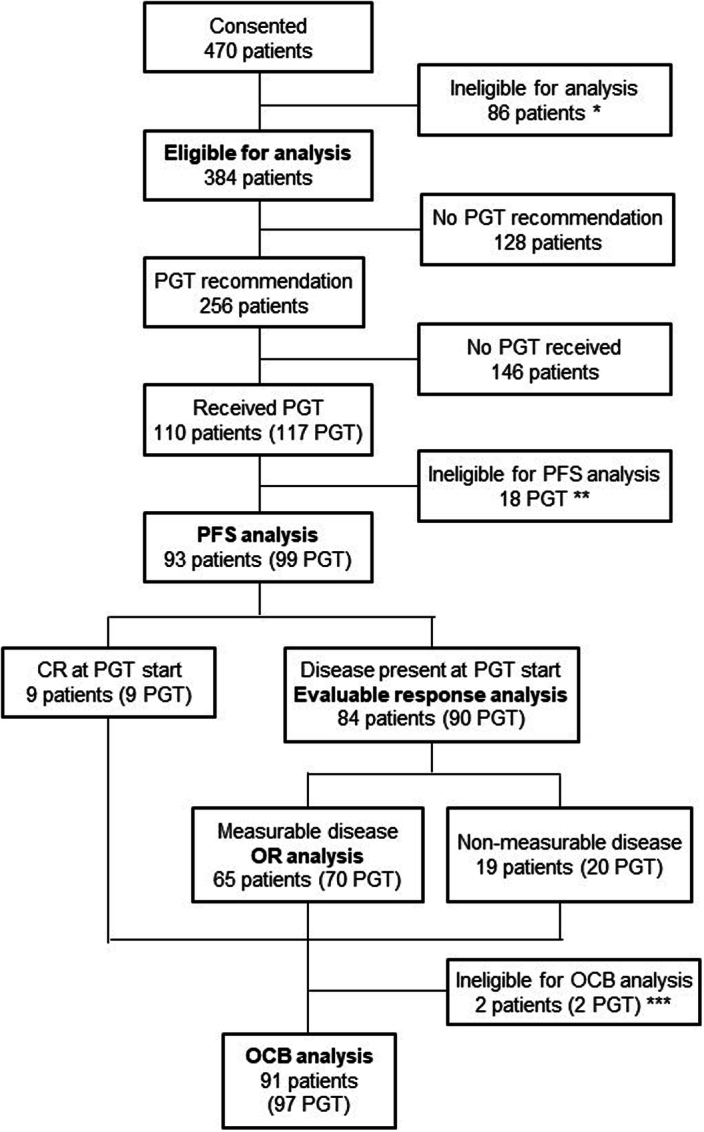
Consort diagram of 470 patients consented in the PRISM study between Sep 2017 and Dec 2020. 384 patients were eligible for outcome analysis. * ineligible due to non-high-risk cancer diagnosis, lack of appropriate sample or death prior to presentation at the molecular tumour board. ** ineligible due to treatment duration <4 weeks, disease progression within the first 4 weeks of treatment or no response evaluation. *** not evaluable for OCB due to cessation of treatment before 24 weeks in the absence of disease progression where stable disease was best response. CR, complete remission; OCB, objective clinical benefit; OR, objective response of measurable disease; PGT, precision-guided treatment; PFS, progression-free survival.
