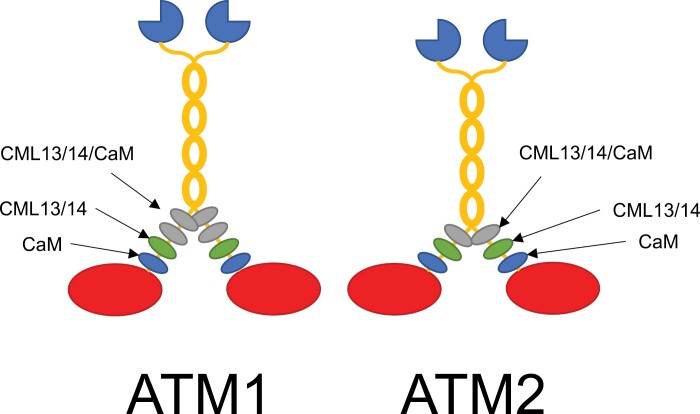
Fig. 9.
Working model of CaM, CML13, and CML14 as light chains for Arabidopsis myosins ATM1 and ATM2. Actin-binding, catalytic myosin head domains are presented in red, the neck regions are decorated with light chains represented as blue (CaM), green (CML13/14), or gray (any of CaM/CML13/14) ovals, the coiled-coil dimerization regions are in yellow, and the cargo-binding tail domains are dark blue, three-quarter circles. Light chains bind to the IQ domains within the neck region of myosins to provide the leverage and structural integrity needed for the power stroke. We speculate that CaM is the preferred light chain at the IQ1 position (nearest the head) for both ATM1 and ATM2, whereas CML13 or CML14 are preferred light chains at the IQ2 position. Other IQ domains did not show a clear preference in our tests and may be occupied by CaM, CML13, CML14, or possibly other light chains.