Figure 2.
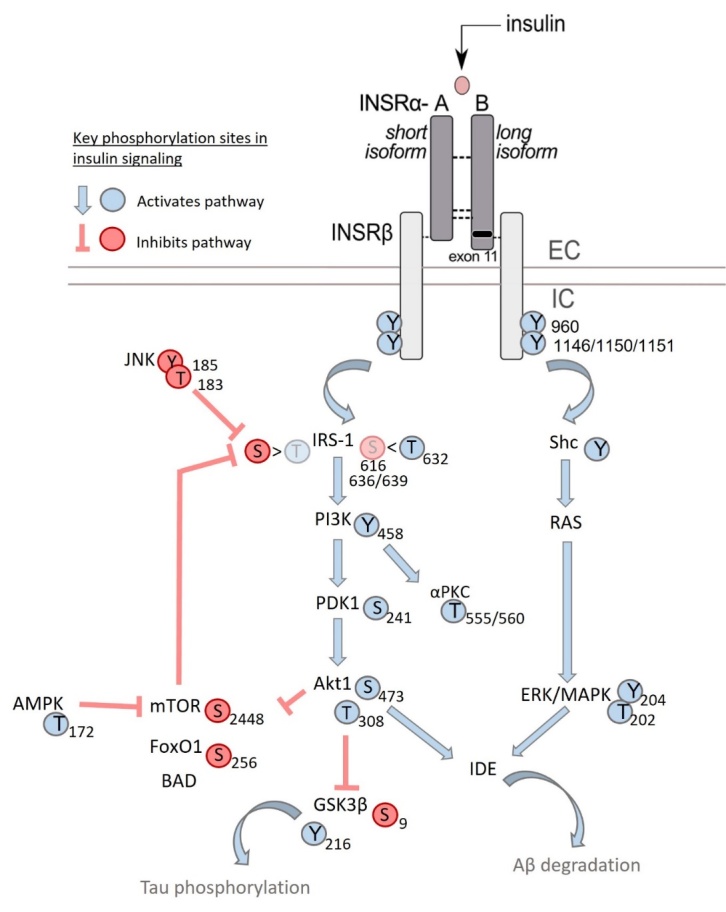
INSR signaling pathway commonly investigated for BIR and phosphorylation sites interrogated. Following insulin binding, the INSR is autophosphorylated at select tyrosine residues, resulting in activation. This triggers activation of the IRS-1 kinase, which can involve either activation on threonine residues or inhibition mediated by serine residues. Threonine phosphorylation of IRS-1 activates the downstream kinases PI3K, PDK1, and Akt1, which all are activated following phosphorylation. Akt activation inhibits GSK3β-mediated tau phosphorylation via tyrosine phosphorylation. Akt1 phosphorylation and subsequent activation can also inhibit mTOR inhibitory phosphorylation. IRS-1 activation can also be inhibited by JNK phosphorylation which limits threonine phosphorylation (activation) and enhances serine phosphorylation (inhibition). The non-canonical INSR signaling pathway involves Shc, Ras, and ERK/MAPK activation. Either Akt or the ERK/MAPK pathway can activate IDE which leads to Aβ degradation. In general, tyrosine and threonine phosphorylation of the kinases present in this pathway elicit activation of the kinase whereas serine phosphorylation inhibits activity of these kinases. Akt, protein kinase B; Aβ, β-amyloid peptide; BAD, Bcl-2-associated death promoter; EC, extracellular; ERK/MAPK, Mitogen-activated protein kinase kinase; FoXO, Forkhead; GSK3β, Glycogen synthase kinase-3 β; IC, intrecellular; INSR, insulin receptor; IRS1, insulin receptor substrates 1; JNK, c-Jun N-terminal kinases; mTor, mammalian target of rapamycin; PI3K, Phosphoinositide 3-kinases; αPKC, atypical protein kinase C.
