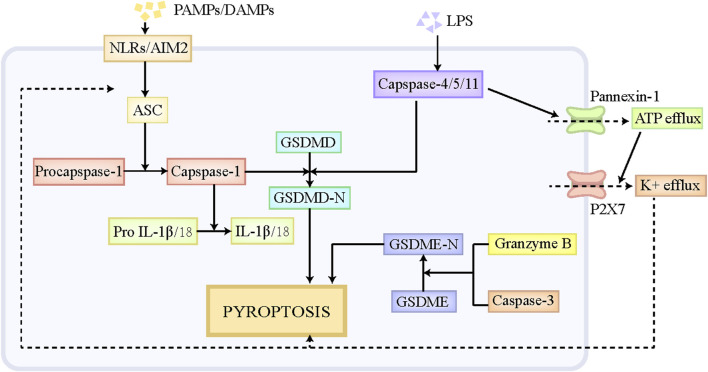
FIGURE 2.
The pathophysiology of pyroptosis. NLRs and AIM2 can recognize endogenous DAMPs and exogenous PAMPs, and then recruits pro-capase-1 by interacting with ASC, thus forming an inflammasome, which cleaves pro-caspase-1 to caspase-1. Caspase-1 subsequently leads to the cleavage of GSDMD and releases its N-terminal fragment, namely GSDMD-N, which ultimately results in pyroptosis. Pro-caspase-4/5/11 has the capacity to directly bind to liposome A at the tail of LPS, which promotes the self-oligomerization and self-activation of caspase-4/5/11. The activated caspase-4/5/11 is able to cleave GSDMD to GSDMD-N and induce pyroptosis. Moreover, Pannexin-1, the channel protein on cellular membrane, also can be cleaved by capsase-11, which then mediates the efflux of ATP. The repeated stimulation of ATP is a potent driver for P2X7 channel opening, which triggers the efflux of K+, Ca+, and Na+, thus causing cell swelling and lytic cell death. In addition, K+efflux also serves as a contributor to activate NLRP3/ASC/caspase-1 signaling axis, further promoting the cleavage of GSDMD. Besides GSDMD, GSDME also play roles in the pathogenesis of pyroptosis. GSDME is cleaved to GSDME-N by granzyme B and caspase-3, which similarly induces the pore formation in cellular membrane and ultimate pyroptosis. Abbreviations: DAMPs, damaged associated molecular patterns; PAMPs, pathogen-associated molecular patterns; NLRs, Nod-like receptors; AIM2, absent in melanoma-2; GSDMD, gasdermin D.