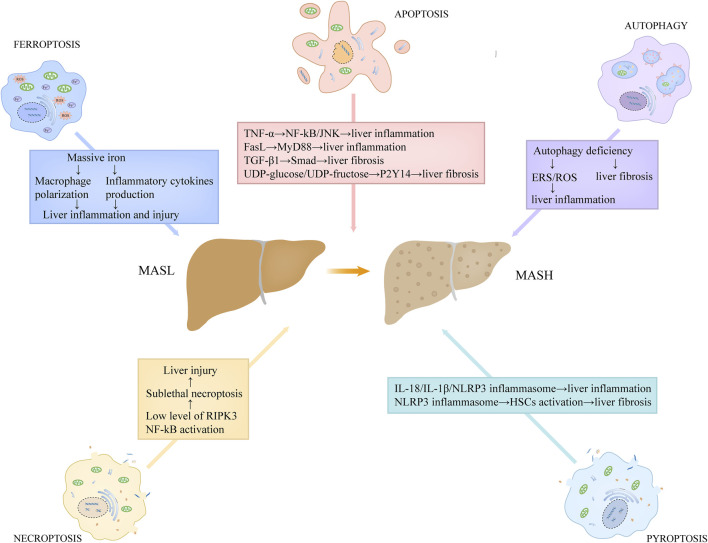
FIGURE 4.
The roles of programmed cell death in the proinflammatory and fibrotic progression in MASH. Hepatic apoptotic bodies derived from apoptotic process stimulate the production of death receptor ligands, such as TNF-α and FasL. TNF-α has the capacity to activate NF-kB and JNK pathways, triggering a series of inflammatory responses. FasL stimulates the secretion of chemokines in macrophages through MyD88 signaling axis. Hepatocyte apoptosis also correlates with the production of TGF-β1 and the release of purinergic ligands, including UDP-glucose and UDP-fructose. Among which, TGF-β1 contributes to liver fibrosis through facilitating extracellular matrix deposition via TGF-β1/Smad axis in HSCs, while UDP-glucose/UDP-fructose recognizes purinergic receptor P2Y14 distributed in HSCs, resulting in the activation of HSCs and liver fibrosis. Low expression of RIPK3 level in hepatocytes and NF-kB activation in MASH may lead to sublethal necroptosis, which expedites the progression of the disease. Pyroptosis induces the release of massive inflammatory cytokines, such as IL-18 and IL-1β, directly promoting inflammatory responses. Moreover, the released NLRP3 inflammasome particles in pyroptosis can be engulfed by HSCs, which results in HSCs activation and α-SMA upregulation, contributing to liver fibrosis. Autophagy deficiency in MASH is unfavorable for the elimination of ERS and dysregulated or impaired mitochondria, thus potentiating ROS generation, which promotes liver inflammation. In addition, hepatocyte autophagy deficiency also contributes to liver fibrosis. Abbreviations: TNF, tumor necrosis factor; MASH, metabolic dysfunction-associated steatohepatitis; HSCs, hepatic stellate cells; NF-kB, nuclear factor-kB; TGF-β1, transforming growth factor-β1; ERS, Endoplasmic reticulum stress; ROS, reactive oxidative stress; RIPK3, receptor-interacting protein kinase 3.