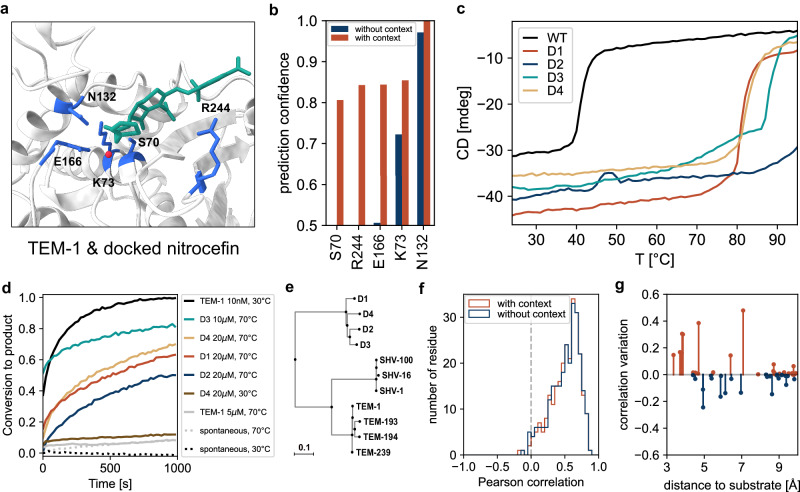
Fig. 4. β-lactamase enzyme engineering and experimental characterization.
a Nitrocefin docked using AutoDock Vina48 at the active site of the serine β-lactamase TEM-1 (PDB ID: 1BT5). Relevant residues for substrate recognition and hydrolysis are shown in blue, nitrocefin in green, and the catalytic water molecule in red. b Prediction confidence with and without the substrate and the catalytic water for the relevant amino acids at the catalytic pocket. (c–d) Experimental characterization of the 4 soluble designs based on the TEM-1 backbone. (c) Thermal denaturation profiles presented as the circular dichroism signal at 222 nm against temperature (see also Supplementary Figs. 10–12 for further structural characterization). d Catalytic activity as fraction of substrate converted to product upon hydrolysis of 200 μM nitrocefin by TEM-1 and the TEM-like lactamase designs, at different temperatures. Proteins were incubated at the indicated concentration (e) Extract of the phylogenetic tree of class A β-lactamases focused on TEM β-lactamases (see Supplementary Fig. 13). f Correlation of the predictions with deep sequencing analysis of TEM-1. g Correlation variation by adding the context (nitrocefin and catalytic water) for the amino acids close (in Cβ distance) to the substrate.