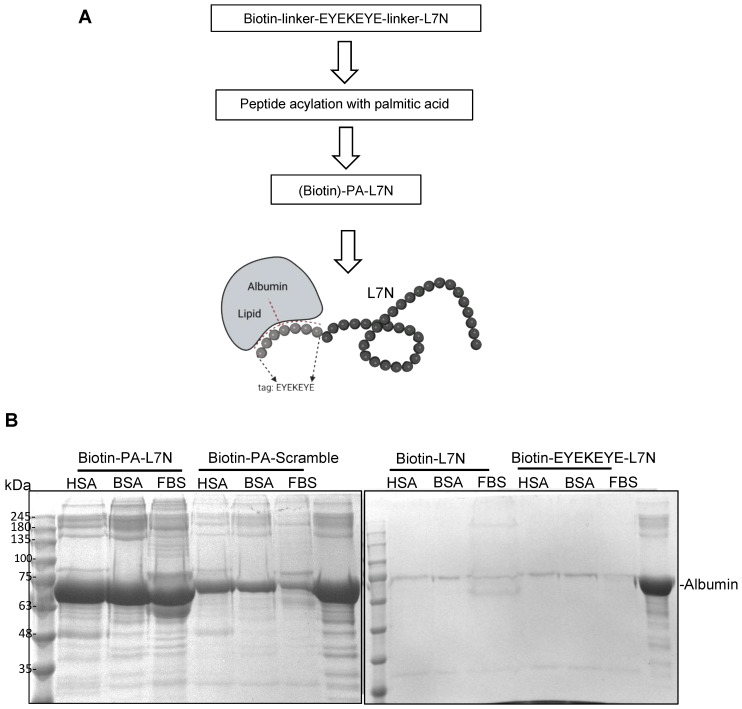
Figure 3.
Stabilization of L7N via generation of an albumin-binding analogue. (A) A schematic showing the main steps taken for synthesizing a palmitic acid–peptide chimera of L7N, or PA-L7N, for albumin binding. An analogue based on scrambled L7N was included as control (PA–scramble). Two types of linkers were used. For pulldown studies, 6-aminohexanoic acid (ahx) was used as the linker, and the peptide was synthesized with a biotin at the N-terminus. Alternatively, a polyethylene glycol linker was used (refer to Table 1 for details). (B) PA-L7N with biotin labeling effectively pulled down albumin in vitro. (Left) Equal concentrations (0.03 mM) of human serum albumin (HSA), bovine serum albumin (BSA), or fetal bovine serum (FBS) were incubated with 1 mM of the biotin–PA-L7N peptide or the biotin–PA–scrambled peptide. Streptavidin beads were used to pull down the biotin peptides, and the bound albumin was detected by SDS-PAGE. (Right) HSA, BSA, and FBS showed no binding to biotin–L7N (with or without the EYEKEYE tag).