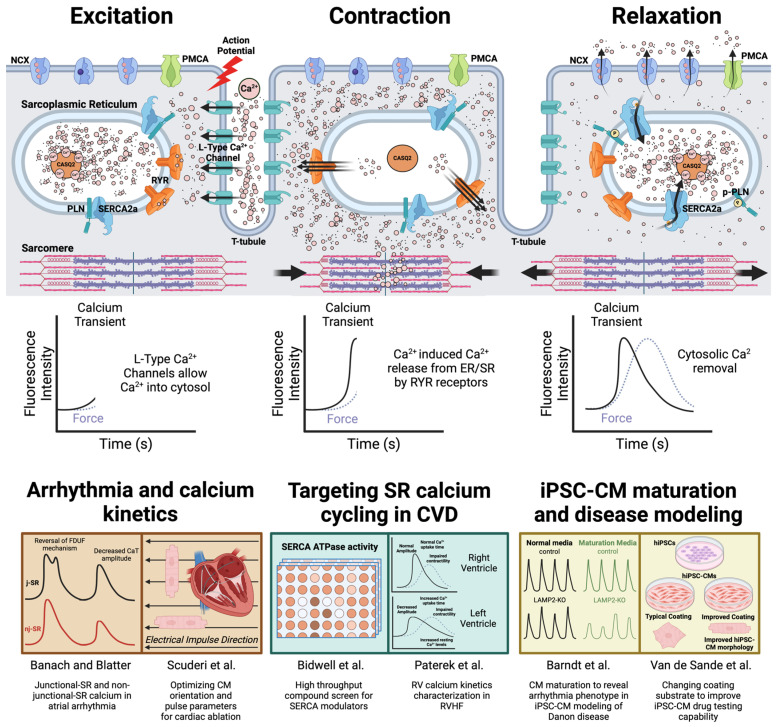
Figure 1.
The application of characterizing cardiomyocyte excitation–contraction coupling in cardiac disease modeling and drug discovery. Membrane depolarization activates L-type calcium channel opening and allows calcium entry into the cardiomyocyte. These calcium ions bind to ryanodine receptors and activate SR calcium release (calcium-induced calcium release, or CICR), which then allows contraction. This calcium release in cardiomyocytes is easily detected by calcium imaging (indicated by the surge in calcium transient signal). Relaxation is enabled by removing cytosolic calcium via SR calcium ATPase (SERCA), plasma membrane sodium–calcium exchange (NCX), and plasma membrane calcium ATPase (PMCA). Calsequestrin (CASQ2) is the major calcium binding protein that absorbs calcium in the SR and increases SR calcium content. Removal of cytosolic calcium can be reflected by the decay phase in calcium imaging. The sequence of calcium cycling is maintained throughout an individual’s lifespan to ensure the continuous pumping action of the heart. Researchers can gather different mechanistic insights related to disease- or chamber-specific cell types by examining this sequence under different research conditions. Six manuscripts published in this Special Issue are summarized here [7,12,15,18,22,30].