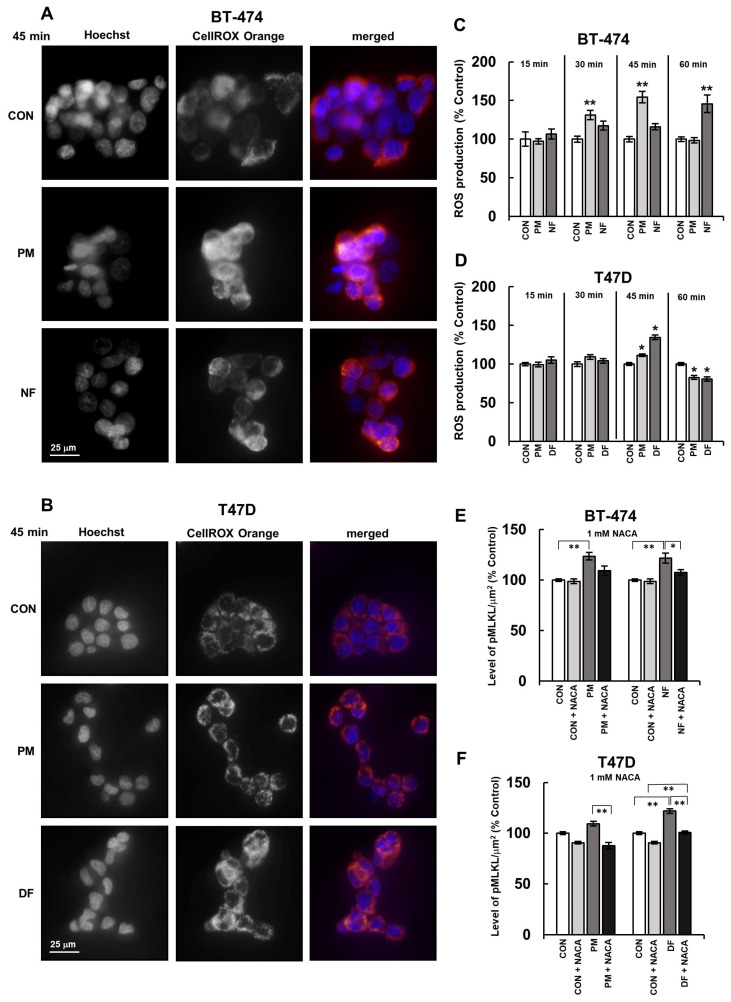
Figure 5.
DS rapidly triggered the redox imbalance and, via this mechanism, induced the activation of MLKL in luminal breast cancer cells. (A,B) Representative images showing ROS production in the BT-474 (A) and T-47D (B) cultures that were exposed to the DS variants (PM–DS from porcine intestinal mucosa and DF–DS from fibrosis-affected human fascia) for the indicated period of time. Then, the cells were stained with Cell ROX Orange (ROS production) and Hoechst (nuclei, blue). (C,D) The dynamics of the DS variant-promoted induction of short-term oxidative stress in the BT-474 (C) and T-47D (D) cells. The cells were exposed to an individual DS variant at a concentration of 25 µM/mL for the indicated periods of time and then treated with Cell ROX Orange. (E,F) The DS-mediated activation of MLKL in the BT-474 (E) and T-47D (F) cells was dependent on this glycan-induced oxidative stress. The activation of MLKL was measured by immunofluorescence in the breast cancer cells that were first preincubated with 1 mM of N-acetylcysteine amide (NACA) for 3 h and then subjected to a combined treatment with an individual DS variant and NACA for 3.5 h. The fluorescence per µm2 was taken from all of the visible cells in six non-overlapping fields from each of the three independent experiments. The results are expressed as the percentage of the fluorescence that was detected in the untreated controls and are presented as the mean ± SEM for all of the obtained images. */**—statistically significant differences (p < 0.05/p < 0.01, respectively) versus the control.