Fig. 2.
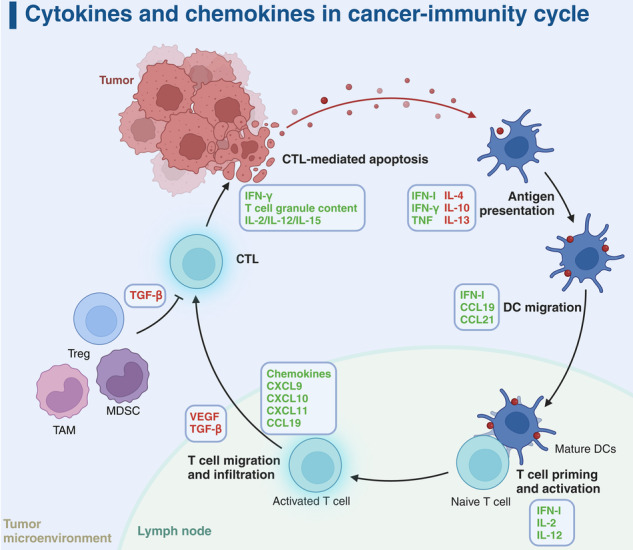
Cytokine dynamics in the cancer-immunity cycle. The figure presents a comprehensive view of cytokine interactions within the cancer-immunity cycle, illustrating the dual role of cytokines in both tumor suppression and promotion. Key features include the promotion of cytotoxic T lymphocyte (CTL)-mediated apoptosis by IFN-γ and various interleukins (IL-2, IL-12, IL-15) within the tumor microenvironment. In contrast, regulatory elements such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) secrete IL-10 and transforming growth factor-beta (TGF-β) to mitigate CTL efficacy and assist in immune evasion. The lymph node emerges as a pivotal site for antigen presentation by dendritic cells (DCs), orchestrated by a suite of cytokines including type I interferon (IFN-I), IFN-γ, tumor necrosis factor (TNF), along with IL-4, IL-10, and IL-13. DC migration to lymph nodes, necessary for T cell priming and activation, is enhanced by IFN-I, chemokine (C-C motif) ligand 19 (CCL19), and CCL21. Subsequently, activated T cells are drawn back to the tumor via a gradient of chemokines, including C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, CXCL11, and CCL19. Nonetheless, the tumor microenvironment, influenced by vascular endothelial growth factor (VEGF) and TGF-β, can counteract T cell infiltration and activation, underscoring the delicate equilibrium between immune defense and tumor immune evasion. Cytokines are distinctly labeled with red and green to denote their immunosuppressive and immunostimulatory functions for antitumor immunity, respectively. Adapted from “Tumor-Specific T Cell Induction and Function”, by BioRender.com (2024). Retrieved from https://app.biorender.com/biorender-templates
