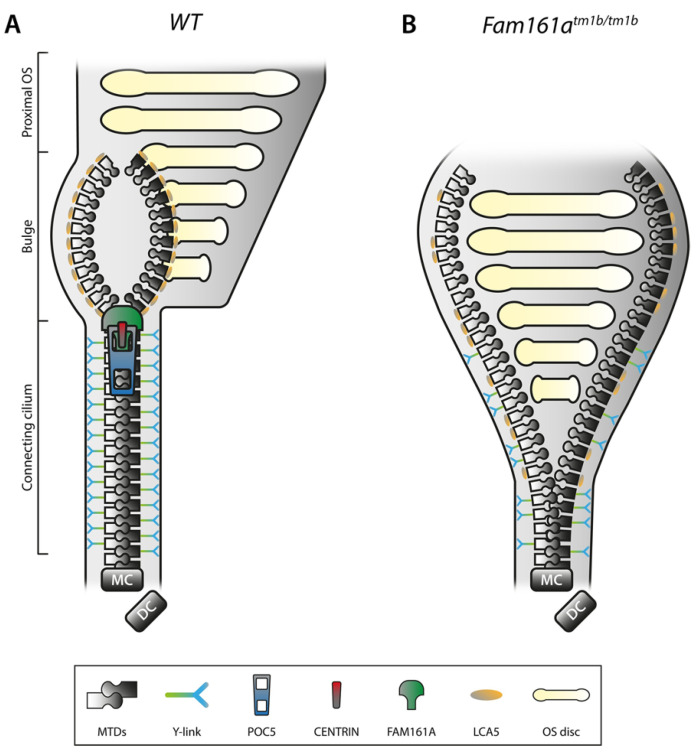
Figure 8.
Schematic representation of the inner scaffold of the connecting cilium within a rod photoreceptor. Taken from Faber and Roepman (2022) [50]. (A) Schematic representation of a part of a WT rod photoreceptor consisting of the CC, the bulge region, and the proximal OS, including its membranous stacked discs. The MTDs are built up from the MC, accompanied by the DC. Cohesion of the MTDs in the CC is maintained by the inner scaffold proteins POC5, CENTRIN and FAM161A, located at the inner wall of the MTDs, comparable with a closed zipper. Note that these proteins are found all along the CC, in addition to the MC and DC. MTDs in the CC are connected to the membrane by Y-links, associated with CEP290 and SPATA7 localization. LCA5 localizes to the bulge region, where MTDs are more dispersed due to the absence of the inner scaffold and Y-links. (B) Deficiency of FAM161A causes loss of the entire zip head (the CC inner scaffold) as also POC5 and Centrin are absent, leading to spreading of the MTDs. This spreading, visualized by an open zipper, eventually causes a collapse of the OS structure. Protein localization at the Y-link level is secondarily affected when FAM161A is depleted, as seen by more dispersed CEP290 localization. Furthermore, FAM161A deficiency results in disorganization of the bulge region, obvious from LCA5 localizing more proximal to the MC. Altogether, the CC inner scaffold forms a structural foundation securing proper disc formation and OS integrity. DC, daughter centriole; CC, connecting cilium; MC, mother centriole; MTD, microtubule doublet; OS, outer segment; and WT, wild-type. FAM161Atm1b/tm1b is mouse model where exon 3 is knocked out, resulting in a short (only exons 1 and 2) non-functioning protein [51].