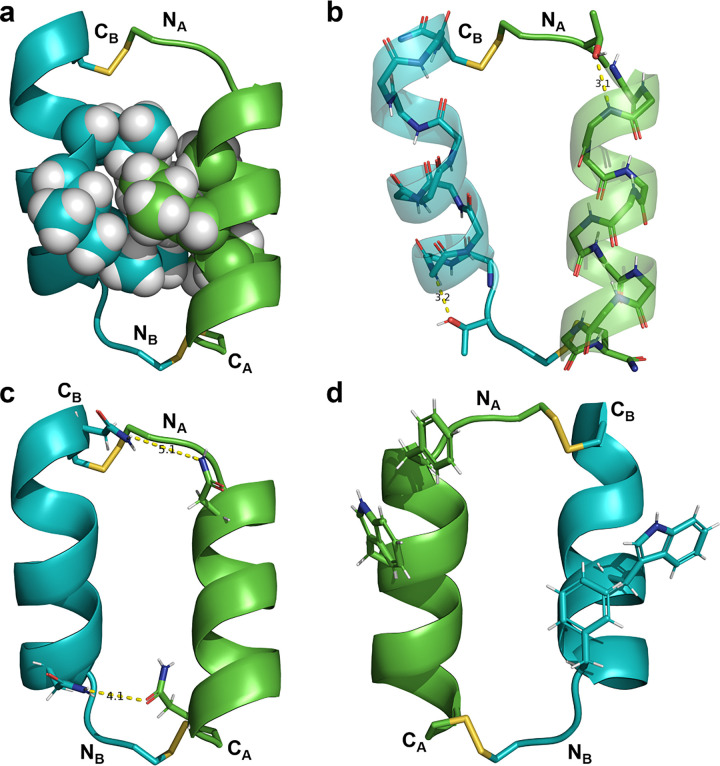
Figure 3.
Crystal structure of disulfide-linked CV1 peptide dimer (PDB 9C5S). In all panels, each helix is represented with the same color (chain A = green, chain B = blue,) and disulfide bonds are represented as yellow sticks. N- and C-termini are labeled as N and C, respectively, with subscripts to indicate the corresponding chain. a) The dimer interface involves interdigitation of the side-chains of Leu6, Leu9, and Leu10 (spheres). b) The oxygen atom of each Thr2 side-chain serves as a hydrogen bond acceptor for Asn5 NH to cap the helix at the N-terminus. c) Asn5 and Asn13 side-chains form long range side-chain-to-side-chain hydrogen bonds between helices. d) Phe3 and Trp7 side-chains (sticks) are peripheral to the helical interface