Abstract
An 80-year-old non-smoking woman was admitted to hospital due to persistent sputum production and dyspnea. She developed respiratory failure, and chest imaging revealed multifocal consolidation and cavities. Her respiratory status did not respond to antimicrobial treatment and progressively worsened, with massive sputum production of approximately 1 L per day, and she died 19 days after admission. The patient was diagnosed with invasive mucinous adenocarcinoma based on a postmortem needle biopsy of the lung. Clinicians should consider invasive mucinous adenocarcinoma in the differential diagnosis of patients who present with massive bronchorrhea and diffuse pulmonary cavity abnormalities.
Keywords: Invasive mucinous adenocarcinoma, Sputum, Bronchorrhea, Cavity, Hepatocyte nuclear factor 4α
Highlights
-
•
Bronchorrhea is defined as the excessive production of sputum of more than 100 mL/day.
-
•
Bronchorrhea is associated with asthma, chronic bronchitis, tuberculosis, and malignant disease.
-
•
Invasive mucinous adenocarcinoma presents with diffuse lung abnormalities associated with bronchorrhea.
1. Introduction
Invasive mucinous adenocarcinoma (IMA) is a specific type of lung adenocarcinoma, in which tumor cells proliferate by replacing the alveolar epithelium and producing abundant mucus [1,2]. IMA is rare and accounts for only 2–5% of all lung adenocarcinomas [3]. The prognosis of IMA is poor because of the difficulties in its diagnosis, resistance to treatment, and a relatively high risk of recurrence [[4], [5], [6]]. Bronchorrhea is a rare condition defined as the excessive production of watery sputum (>100 mL per day) [1,7]. Few patients with bronchorrhea produce >500 mL of sputum per day [7]. We present a rare case of rapidly progressive IMA with massive sputum production of approximately 1 L per day.
2. Case presentation
An 80-year-old non-smoking woman presented with a 6-month history of sputum production, cough, and dyspnea. The patient had been receiving methotrexate for rheumatoid arthritis for several years. On admission, physical examination revealed a body temperature of 38.2 °C, oxygen saturation of 87 % on ambient air, heart rate of 86 beats/min, blood pressure of 134/69 mmHg, and respiratory rate of 18/min. Lung auscultation revealed coarse crackles bilaterally. Chest radiography revealed consolidation in the right lower lung field and patchy ground-glass opacities (GGOs) in both lung fields (Fig. 1A). Chest computed tomography (CT) showed multifocal consolidation with GGOs and cavitation, predominantly in the lower lobes (Fig. 2).
Fig. 1.
Chest radiograph on admission showing consolidation in the right lower lung field and patchy ground-glass opacities in both lung fields (A). Lung involvement worsened 10 days after admission (B).
Fig. 2.
Chest computed tomography on admission showing multifocal consolidation with cavities and surrounding ground-glass opacities, predominantly in the lower lobes.
The patient's laboratory test results were as follows: white blood cell count 8300/mL with 84.8 % neutrophils; hemoglobin 11.6 g/dL; platelets 34.5 × 104/μL; lactate dehydrogenase 437 U/L, C-reactive protein 9 mg/dL; β-D-glucan <5 pg/mL (normal value, <20 pg/mL); cryptococcal antigen negative, interferon-gamma release assay negative, soluble interleukin-2 receptor 803 U/mL, Krebs von den Lungen-6 163 U/mL (normal value, <499 U/mL), proteinase 3/myeloperoxidase-antineutrophil cytoplasmic autoantibody negative, and carcinoembryonic antigen 4.1 ng/mL (normal value, <5 ng/mL). An arterial blood gas analysis under 3 L/min oxygen administration showed a pH of 7.48, PaO2 of 70 Torr, PaCO2 of 42 Torr, and HCO3− of 31.3 mmol/L. Antimicrobial treatment with ceftriaxone (2 g/day) was initiated, but the lung involvement rapidly worsened (Fig. 1B) along with the respiratory status. Ten days after admission, mechanical ventilation was initiated, and measurement showed that more than 1000 mL of yellow-white, viscous sputum was aspirated daily through the tracheal tubce (Fig. 3). A sputum examination revealed no bacterial or acid-fast bacilli. The repeated cytological analysis of the sputum indicated no evidence of malignancy. Thereafter, the patient's respiratory status did not improve, with massive expectoration of sputum, and the patient died 19 days after admission.
Fig. 3.
The yellow-white sputum aspirated through the tracheal tube. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
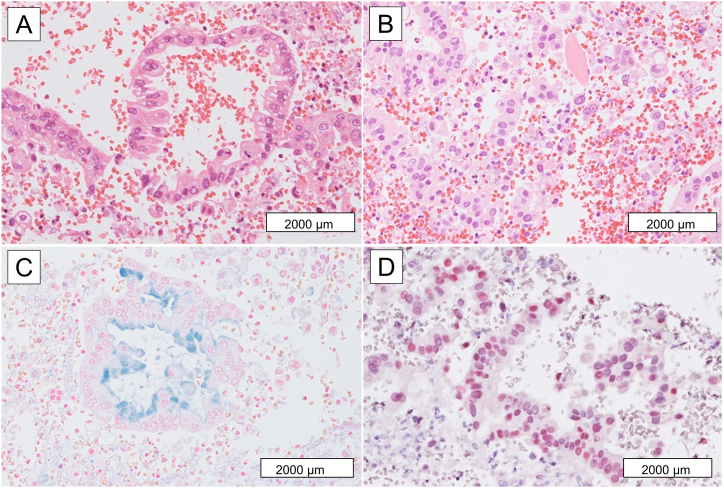
A postmortem lung biopsy (needle necropsy) revealed the proliferation of atypical columnar cells with occasional intracytoplasmic mucin (Fig. 4). The atypical cells were diffusely positive for cytokeratin 7, cytokeratin 20, and hepatocyte nuclear factor 4α (HNF4α) but negative for thyroid transcription factor-1 (TTF-1), Napsin A, CDX2, MUC5AC and MUC6. Based on the histological and immunohistochemical findings, IMA of the lung was diagnosed.
Fig. 4.
Needle necropsy of the lung showing the proliferation of atypical columnar cells with occasional intracytoplasmic mucin, associated with degenerative changes (A, C Hematoxylin and Eosin staining, B, Alcian blue staining). Atypical cells are diffusely positive for hepatocyte nuclear factor 4α (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
Normal human lungs produce approximately 10–100 mL of mucus per day to support mucociliary clearance [7]. Postnasal drip, asthma, gastroesophageal reflux disease, and bronchitis are frequent causes of excessive sputum production [7]; however, the amount of sputum expectoration is usually less than 25 mL per day, even under these conditions [8]. Bronchorrhea is associated with lung diseases such as asthma, chronic bronchitis, tuberculosis, and malignancy [8]. Among these diseases, IMA is a relatively common cause of bronchorrhea [9]. The amount of sputum in patients with IMA complicated with bronchorrhea is relatively larger than that in patients with bronchorrhea associated with other diseases, yet it rarely exceeds 500 mL/day [7,10]. Corticosteroids, macrolides, inhaled indomethacin, octreotide, and tyrosine kinase inhibitors have been reported as treatments for bronchorrhea, but none has proven to be effective [11]. Although chemotherapy is a treatment option for patients with IMA-related bronchorrhea, mucus production usually increases or remains largely unchanged despite anti-tumor treatment [11].
This case was unique because the multifocal consolidation with surrounding GGO and cavitation resembled bacterial pneumonia or septic embolism. Lung cavities can arise from a variety of underlying diseases, including infectious diseases, such as pulmonary tuberculosis and aspergillosis, and non-infectious diseases, such as lung cancer and granulomatosis with polyangiitis [12]. Chest CT images of patients with IMA sometimes show cavitation or pseudocavitation, which is generally more consistent with respiratory infection or septic embolism than with lung cancer [13]. Pseudocavitation is defined as a rounded lesion of low attenuation less than 1 cm in diameter that occurs within consolidation [14]. The presence of pseudocavitation is associated with a higher likelihood of adenocarcinoma with a lepidic growth pattern that seen in IMA [14]. Cavitation in lung cancer is thought to be caused by several mechanisms, including disruption of normal alveolar walls due to tumor invasion or the release of proteolytic enzymes, the check valve mechanism, and necrosis due to lack of blood supply [13]. Since IMA is generally considered to be less prone to necrosis, the check-bulb mechanism is the most likely explanation for the formation of cavitary lesions [15]. Some histologic analyses have shown air-filled cysts with increased size and wall thickness and the presence of an intraluminal mucus plug in the conductive airway in patients with IMA [15,16].
The present case was characterized by rapid progression and a short survival time. Previous reports have shown that it takes 8–11 months to diagnose IMA, which is much longer in comparison to other types of lung cancer [6]. An antemortem diagnosis is sometimes difficult to make, especially in patients presenting with bronchorrhea or respiratory failure. One of the reasons for the difficulty in diagnosing IMA is the inconspicuous or absent cytological atypia in tumor cells [6]. Immunohistochemically, tumor cells in the IMA often do not express TTF-1 but express HNF4α, a nuclear transcription factor essential for goblet cell maturation, which provides a strong basis for the diagnosis [17,18]. Thus, HNF4α positivity could serve as a useful marker to overcome diagnostic difficulties, especially in small samples [18]. Meanwhile, a recent study reported that the diffuse expression of MUC6 was observed in 27 % of patients with IMA and was associated with favorable outcomes [17]. Mucins are involved in a wide range of biological activities, and the expression of specific types of mucins can suppress cancer cell progression [17,19]. In the present case, the tumor cells were negative for MUC6, which may indicate a progressive IMA phenotype.
4. Conclusion
We herein report an instructive and educational case of IMA with massive bronchorrhea and progressive respiratory failure. Although extensive lung opacities and pronounced sputum production are rare in IMA, clinicians should consider IMA in the differential diagnosis of diffuse pulmonary abnormalities associated with bronchorrhea.
Financial/nonfinancial disclosures
None declared.
Funding information
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Notation of prior abstract publication/presentation
None declared.
Patient consent for publication
Obtained.
CRediT authorship contribution statement
Kengo Takata: Writing – review & editing, Writing – original draft, Investigation, Data curation, Conceptualization. Yoshiaki Kinoshita: Writing – review & editing, Writing – original draft, Supervision, Investigation, Data curation. Masayo Yoshimura: Writing – review & editing. Shota Takenaka: Writing – review & editing, Data curation. Takuhide Utsunomiya: Writing – review & editing, Data curation. Yohei Koide: Writing – review & editing, Data curation. Kenji Wada: Writing – review & editing, Data curation. Yuji Yoshida: Writing – review & editing, Data curation. Shota Nakashima: Writing – review & editing, Data curation. Hisako Kushima: Writing – review & editing, Data curation. Satoshi Nimura: Writing – review & editing. Hiroshi Ishii: Writing – review & editing, Writing – original draft, Supervision, Project administration, Conceptualization.
Declaration of generative AI and AI-assisted technologies in the writing process
The author did not use generative AI or AI-assisted technologies during the preparation of this work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kurahara Y. Massive bronchorrhea. Intern. Med. 2021;60:2155–2156. doi: 10.2169/internalmedicine.6602-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis W.D., Brambilla E., Noguchi M., Nicholson A.G., Geisinger K.R., Yatabe Y., Beer D.G., Powell C.A., Riely G.J., Van Schil P.E., Garg K., Austin J.H.M., Asamura H., Rusch V.W., Hirsch F.R., Scagliotti G., Mitsudomi T., Huber R.M., Ishikawa Y., Jett J., Sanchez-Cespedes M., Sculier J.-P., Takahashi T., Tsuboi M., Vansteenkiste J., Wistuba I., Yang P.-C., Aberle D., Brambilla C., Flieder D., Franklin W., Gazdar A., Gould M., Hasleton P., Henderson D., Johnson B., Johnson D., Kerr K., Kuriyama K., Lee J.S., Miller V.A., Petersen I., Roggli V., Rosell R., Saijo N., Thunnissen E., Tsao M., Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H.Y., Cha M.J., Lee K.S., Lee H.Y., Kwon O.J., Choi J.Y., Kim H.K., Choi Y.S., Kim J., Shim Y.M. Prognosis in resected invasive mucinous adenocarcinomas of the lung: related factors and comparison with resected nonmucinous adenocarcinomas. J. Thorac. Oncol. 2016;11:1064–1073. doi: 10.1016/j.jtho.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Regnard J.F., Santelmo N., Romdhani N., Gharbi N., Bourcereau J., Dulmet E., Levasseur P. Bronchioloalveolar lung carcinoma: results of surgical treatment and prognostic factors. Chest. 1998;114:45–50. doi: 10.1378/chest.114.1.45. [DOI] [PubMed] [Google Scholar]
- 5.Cha Y.J., Shim H.S. Biology of invasive mucinous adenocarcinoma of the lung. Transl. Lung Cancer Res. 2017;6:508–512. doi: 10.21037/tlcr.2017.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumi T., Nagayama D., Suzuki K., Koshino Y., Ikeda T., Watanabe H., Yamada Y., Terai K., Chiba H. Invasive mucinous adenocarcinoma diagnosed using transbronchial cryobiopsy. Respir. Endosc. 2023;1:119–122. doi: 10.58585/respend.2023-0038. [DOI] [Google Scholar]
- 7.Smyrnios N.A., Irwin R.S., Curley F.J. Chronic cough with a history of excessive sputum production. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Chest. 1995;108:991–997. doi: 10.1378/chest.108.4.991. [DOI] [PubMed] [Google Scholar]
- 8.Keal E.E. Biochemistry and rheology of sputum in asthma. Postgrad. Med. 1971;47:171–177. doi: 10.1136/pgmj.47.545.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhagat M., Singh A., Bazzi T., Green J. Bronchorrhea, a rare and debilitating symptom of lung cancer: case report and review of the treatment. JTO Clin. Res. Rep. 2022;3 doi: 10.1016/j.jtocrr.2022.100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Żylicz Z. The challenge of bronchorrhea in advanced cancer — a case report with review of literature. Adv. Palliat. Med. 2010;9:9–11. https://journals.viamedica.pl/advances_in_palliative_medicine/article/view/29369 [Google Scholar]
- 11.Rémi C., Rémi J., Bausewein C. Pharmacological management of bronchorrhea in malignant disease: a systematic literature review. J. Pain Symptom Manag. 2016;51:916–925. doi: 10.1016/j.jpainsymman.2015.12.335. [DOI] [PubMed] [Google Scholar]
- 12.Wasim Jamal S.M., Aboukamar M.R., Khatib M., Al Maslamani M., Nashwan A.J. Lung cancer mimicking aspergilloma: a case report, case rep. Oncol. 2023;16:1318–1323. doi: 10.1159/000534527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuzawa K., Minematsu N., Sasaki M., Ohsawa K., Yamamoto T., Iwamaru A., Ogata K., Betsuyaku T., Murakami M. Invasive mucinous adenocarcinoma of the lung presenting as a large, thin-walled cyst: a case report and literature review. Mol. Clin. Oncol. 2017;6:433–437. doi: 10.3892/mco.2017.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utrera Pérez E., Trinidad López C., González Carril F., Delgado Sánchez-Gracián C., Villanueva Campos A., Jurado Basildo C. Can pseudocavitation in lung tumors predict the diagnosis of adenocarcinoma with lepidic growth? Radiologia. 2019;61:396–404. doi: 10.1016/j.rx.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Lee P., Weerasuriya D.K., Lavori P.W., Quon A., Hara W., Maxim P.G., Le Q.-T., Wakelee H.A., Donington J.S., Graves E.E., Loo B.W., Jr. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:328–333. doi: 10.1016/j.ijrobp.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Kawabata Y., Hoshi E., Takayanagi N., Sugita Y. [Pathological study of the natural history of pulmonary infarction mainly seen in lung tumors--pulmonary infarction begins with alveolar wall bleeding] Nihon Kokyuki Gakkai Zasshi. 2009;47:851–857. https://www.ncbi.nlm.nih.gov/pubmed/19882905 [PubMed] [Google Scholar]
- 17.Kishikawa S., Hayashi T., Saito T., Takamochi K., Kohsaka S., Sano K., Sasahara N., Sasa K., Kurihara T., Hara K., Suehara Y., Takahashi F., Suzuki K., Yao T. Diffuse expression of MUC6 defines a distinct clinicopathological subset of pulmonary invasive mucinous adenocarcinoma. Mod. Pathol. 2021;34:786–797. doi: 10.1038/s41379-020-00690-w. [DOI] [PubMed] [Google Scholar]
- 18.Sugano M., Nagasaka T., Sasaki E., Murakami Y., Hosoda W., Hida T., Mitsudomi T., Yatabe Y. HNF4α as a marker for invasive mucinous adenocarcinoma of the lung. Am. J. Surg. Pathol. 2013;37:211–218. doi: 10.1097/PAS.0b013e31826be303. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia R., Gautam S.K., Cannon A., Thompson C., Hall B.R., Aithal A., Banerjee K., Jain M., Solheim J.C., Kumar S., Batra S.K. Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev. 2019;38:223–236. doi: 10.1007/s10555-018-09775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]