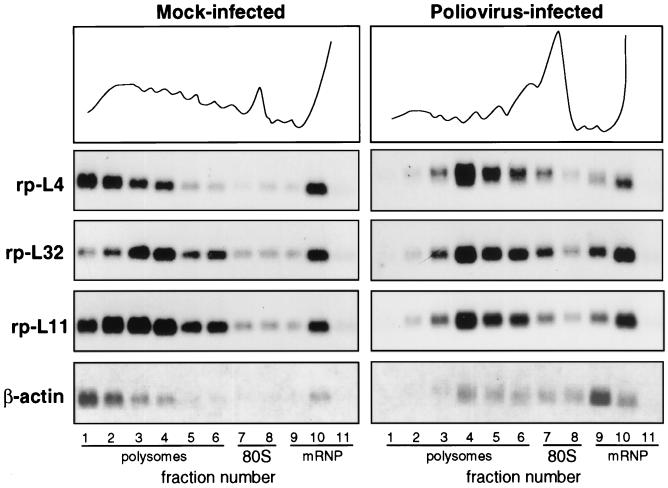
FIG. 1.
Polysome-mRNP distribution of mRNA in mock-infected and poliovirus-infected cells. HEp-2 cells were grown at 37°C in Eagle’s minimum essential medium supplemented with 10% fetal calf serum. When cells reached 80% confluence, the Mahoney strain of poliovirus type 1 was added at a multiplicity of infection of 50 PFU per cell. A 9-cm-diameter plate culture of mock-infected and infected cells was lysed at 4 h postinfection (10 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 1% Na-deoxycholate, 1 mM dithiothreitol, 10 mM Tris-HCl, pH 7.4) to prepare cytoplasmic extracts (32). Cycloheximide, often used to prevent polysome runoff, was not added prior to extract preparation, as this drug sometime creates problems (38). Polysome protection can also be obtained by quickly preparing the extract and loading the sample on the gradient under strict temperature control. The extracts were separated on 15 to 50% sucrose gradients in gradient buffer (0.1 M NaCl, 10 mM MgCl2, 30 mM Tris-HCl, pH 7). Gradient fractions were collected while the optical density profile at 260 nm was monitored (top), and the RNA was prepared by protease K-SDS-phenol extraction (32) of the fractions. The RNAs from equal volumes of mock-infected and infected gradient fractions were loaded onto two gels and analyzed by Northern blotting and autoradiography as previously described (32). Each filter was subsequently hybridized to probes for rp-L4 (2), rp-L32 (10), rp-L11 (12), and β-actin (6) mRNAs to obtain a reliable comparison of the distribution of the various RNAs along the same gradient. Probes were prepared by the random primer technique.