Abstract
Background
Quetiapine (QET) abuse has increased due to its anxiolytic and hedonic effects, necessitating protective adjunct treatments. Acacia saligna (A. saligna) flowers, used in traditional medicine, have potential health benefits.
Aim
To investigate the protective role of A. saligna flower extract against QET-induced sexual toxicity, and to elucidate the possible underlying mechanisms through metabolomic and physiological studies.
Methods
A. saligna extract was subjected to metabolite profiling via High-Resolution Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-ESI–qTOF-MS). Forty-eight adult male albino rats were assigned into six groups for 30 days. The intracavernosal pressure (ICP), semen, biochemical, hormonal, histological, genetic and Western blot (WB) analyses were determined.
Results
A. saligna extract is rich in phenolic compounds, flavonoids, tannins, and unsaturated fatty acids. QET significantly decreased ICP and negatively affected semen parameters. A. saligna mitigated decreased sperm motility and ameliorated overexpressed proinflammatory genes in QET-55 group. A. saligna ameliorated the reduction of the antioxidant biomarkers, testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH), concurrent with downregulation of the nuclear factor kappa B (NF-κB) protein. A. saligna counteracted the disrupted testicular and prostatic structures revealed by histological examination.
Conclusion
The extract from A. saligna, which contains a high concentration of antioxidants and anti-inflammatory chemicals, effectively mitigates sexual toxicity caused by QET. This study provided the first known explanation of the hypothesized processes behind the protective properties of A. saligna through biological, biochemical, and histological parameters. The results emphasize the potential of A. saligna as a safeguarding agent against drug-induced sexual toxicity.
Keywords: Acacia saligna, UPLC-MS/MS, Quetiapine, Sexual toxicity, Oxidative stress, P13K/NF-κB pathway
Abbreviations
- A. Saligna
Acacia saligna
- CAT
Catalase
- FSH
Follicle-stimulating hormone
- GSH
Glutathione
- H&E
Hematoxylin and Eosin
- ICP
Intracavernosal Pressure
- LH
Luteinizing hormone
- MAP
Mean Arterial Pressure
- MDA
Malondialdehyde
- NE
Eosin-Nigrosin stain
- NF-κB
Nuclear Factor kappa B
- NO
nitric oxide
- Quetiapine:
QET
- SOD
Superoxide Dismutase enzyme
- TAC
Total antioxidant capacity
- UPLC-ESI–qTOF-MS
High-Resolution Ultra-Performance Liquid Chromatography-Mass Spectrometry
- WB
Western Blot
1. Introduction
Psychoactive drug use has increased over the last few decades due to psychiatric disorder prevalence [1]. Antidepressants, antipsychotics, and mood stabilizers are routinely given psychoactive [2]. Erectile dysfunction, ejaculation delay, and lower libido result from these medicines [3]. Psychoactive medicines may lower fertility and sperm quality [[4], [5], [6], [7]].
Quetiapine is a second-generation antipsychotic drug. It is prescribed to treat schizophrenia and bipolar diseases [8]. Additionally, it became a treatment for depression, anxiety, and sleep disturbances [[9], [10], [11]]. Off-label prescribing, which includes prescribing QET outside of approved indications for titration in schizophrenia treatment of insomnia and generalized anxiety disorder, has led to an increase in QET misuse [12]. Several reports have associated the second-generation antipsychotic (SGA) quetiapine with misuse and dependency [13]. Quetiapine overdose increased by 90 % between 2005 and 2011, as shown in US reports [14]. Similarly, in Australia, there was an 8-fold increase in quetiapine-associated cases between 2001 and 2010 [15]. There is rising evidence that quetiapine is abused for its anxiolytic, hedonic effects [16]. Both quetiapine and its metabolite norquetiapine have been found to increase levels of the anti-inflammatory cytokine IL-10 and decrease levels of the pro-inflammatory cytokine IFN-γ in the blood 4 h after LPS administration [17]. While many studies have highlighted the beneficial anti-inflammatory role of quetiapine in neurological diseases such as schizophrenia and anhedonia [[17], [18], [19]], other research has revealed its toxic and pro-inflammatory effects on organs such as the heart and gonads [[20], [21], [22]]. The cytochrome P450 enzyme system plays a crucial role in the metabolism of antipsychotic drugs, with individuals with unfavorable genotypes being more susceptible to side effects. Future studies are needed to validate these findings and understand the relationship between the antipsychotic response and side effects [20]. Antipsychotic treatment can cause brain volume loss and astrocyte death, but the mechanisms remain unclear. According to a study, quetiapine, risperidone, and haloperidol made astrocytes much less viable and increased the expression of NLRP3, caspase-1, caspase-4, and GSDMD proteins [23]. Hearts treated with quetiapine displayed signs of inflammation and clear fibrosis following continuous injection for 21 days. The quantified elevations in protein levels of RIP3, MLKL, and the phosphorylation of MLKL indicate that quetiapine triggers necroptotic cell death in both in vivo and in vitro settings. The selective inhibitor Necrostatin-1 was used to pharmacologically block necroptosis and reduce quetiapine-induced cardiac damage in rats [21]. Moreover, QET treatment led to a decline in sperm quality, disrupted hormone levels, and triggered oxidative stress [24].
In recent times, there has been a notable global surge in the utilization of herbal drugs or natural products as therapeutic remedies for various ailments [25,26]. A. saligna plants (Family: Fabaceae or Leguminosae), ≈1350 sp., are widely used in traditional medicines in many countries for the treatment of diarrhea, diabetes, obesity, irritable bowel, hemorrhoids, and wounds, alongside eye and skin ailments (Rather et al., 2015, [27]). A. saligna is native to Western Australia. It is present in Saudi Arabia, Egypt, and other Mediterranean countries in Africa [28,29]. These herbs are chemically interesting via their richness with many classes of bioactive metabolites, especially flavonoids, tannins, phenolic acids, saponins, alkaloids, terpenes, and essential oils (Rather et al., 2015 [27]; El Gendy et al., 2022).
Several studies described different biological and pharmaceutical potentialities of the different extracts of A. saligna such as anti-ulcerative colitis [27], antioxidant [30], cytotoxic ([29], Gedara and Galal, 2014), antimicrobial (Gumgumjee and Hajar, 2015, [30]), and anti-hyperglycaemic [31] in addition to allelopathic effects (Jeddi et al., 2022). Various classes of compounds were characterized from the extracts of A. saligna, including flavonoids, phenolic acids, spirostane saponins, and lignans ([27,29]; Gedara and Galal, 2014). The primary objective of a previous study was to examine the impact of replacing half or all of the clover hay with tannin-rich A. saligna leaves on the reproductive and productive abilities of ewe lambs. The examined groups' conception rates did not differ, but the A. saligna-treated group's fertility and lambing rates were higher than those of the control [32]. In another study, A. saligna-based diets were tested on plasma testosterone and semen characteristics in eight-week-old rabbits. Compared to the control, rabbits fed varied amounts of A. saligna had no significant changes in libido, ejaculate volume, sperm concentration, or packed sperm volume. Low or medium A. saligna levels significantly increased sperm output, motility, and plasma testosterone. High A. saligna levels lowered live and normal sperm but did not impact sperm motility (%) or testosterone [33].
NF-κB signaling, a transcription factor involved in inflammatory responses, has been identified as a pivotal pathway in various disease states [34,35]. The serine/threonine kinase Akt, also known as PKB, is activated by lipid products of phosphatidylinositol 3-kinase (PI3K) and regulates a broad cascade of target proteins, including nuclear factor-κB (NF-κB) [36]. NF-κB is transcriptionally regulated by TNFα and IL-1β, which mediate various inflammations and cellular immune responses [37]. A study found that six flavonoids and nine phenolic compounds in the extract from A. saligna, including naringenin 42, taxifolin 41, (+) catechin 14, quercetin 3, rutin 6, and kaempferol 22, have antioxidant and anti-inflammatory activities. These compounds inhibit COX-2, IL-1, and NF–B activities, while rutin 6 can down-regulate these activities [38].
To the best of our knowledge, there has been no previous research evaluating the mitigating effects of A. saligna on reproductive toxicity caused by quetiapine (QET). The purpose of this study is to propose that the extract from A. salignaflowers has the potential to improve sexual toxicity caused by QET, based on its anti-inflammatory and antioxidant capabilities. In addition, our objective was to assess the therapeutic benefits of A. saligna in mitigating the adverse effects caused by two distinct dosages of QET, in order to ascertain its efficacy in treating reproductive system damage at different levels. The aims of this work were to: i) analyze the hydroethanolic extract ofA. saligna flowers using UPLC-MS-MS, and ii) evaluate the protective effects of A.saligna flower extract against QET-induced toxicity on male rat sexual functioning.
2. Materials and methods
2.1. Collection of A. saligna flowers and extraction procedure
The fresh flowers of A. saligna were collected during the flowering period at the end of April 2021 from Gamasa (31.5280741 E, 31.4293955 N), Kafr El-Sheik governorate, Egypt. The collection started at 5:00 a.m. and ended before sunrise. The plant was authenticated by a taxonomy professor, and kept in the herbarium of Mansoura University, with the following record (AS(x2871)-xR-17533-021) assigned. Flowers were dried for ten days, left in the shadow and open-air room until they finished drying, and ground to very fine powder. A. saligna powdered flowers (850 g) were macerated in 70 % hydro-ethanol (3 L) for five days at ± 25 °C and then filtered. This step was repeated three times, and all extracts were collected and dried under a reduced vacuum, yielding dark brown gum (23.5 g) that was stored in the refrigerator at four °C until further analysis.
2.2. Chemical analysis via High-Resolution Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-ESI–qTOF-MS)
Using a 70 % hydro-ethanol solution, 1 gm of powdered A. saligna was extracted for 1 h using an ultrasonic bath (Branson Ultrasonic Corporation, Danbury, CT, USA). The extract was then filtered and centrifuged for 15 min. After that, the clear supernatant from the extract liquid was separated and analyzed using UPLC-ESI-QTOF-MS. The UPLC-ESI-QTOF-MS procedures were carried out according to the protocol published by Kabbash (2023) and Kalled et al. (2019). In brief, 20 min of sonication and frequent shaking were used to extract 10 mg of the dried, finely ground plant sample using 100 % MeOH (2 mL) and umbelliferone (10 g/mL−1) as an internal standard. The debris was eliminated by centrifuging at 12000×g for 10 min. Subsequently, a C18 cartridge was utilized to do solid-phase extraction on the 22-μm-filtered extract. Next, a volume of 2 μL of the plant extract was introduced into an HSS T3 column (100 × 1.0 mm, particle size 1.8 μm; Waters), which was set up on an ACQUITY UPLC system (Waters, Milford, MA, USA) that was outfitted with a 6540 Ultra-High-Definition (UHD) Accurate-Mass qTOF-LC-MS (Agilent, Palo Alto, CA, USA) connected to an ESI interface and capable of being operated in any direction. By creating a potential formula with a mass accuracy limit of 10 ppm, accounting for tandem MS2 data, RT, literature reviews, and the Phytochemical Dictionary of Natural Products Database, the metabolites were identified [39,40].
2.3. Animals
A total of 48 Albino Wistar rats, aged 10–12 weeks and weighing 200–250 g, were sourced from the animal house at the Faculty of Veterinary Medicine, Suez Canal University, Egypt. The rats were accommodated in spacious plastic cages with five rats per cage, situated in the Animal House at the Faculty of Medicine, Suez Canal University. They were provided ad libitum access to a standard rat chow diet and tap water, and allowed to acclimate for one week prior to commencing the study. All experimental procedures adhered to established protocols and International Guidelines for the Care and Use of Laboratory Animals. Approval for the study was obtained from the Ethical Committee of the Faculty of Medicine, Suez Canal University, under the authority acceptance number of 4904.
2.4. Chemicals
QET (Seroquel 25 mg tablets, AstraZeneca, US) and Thiopental Na in white powder form (500 mg, vial) were supplied by Epico Pharmaceutical Company, 10th of Ramadan City, Egypt.
2.5. Experimental design
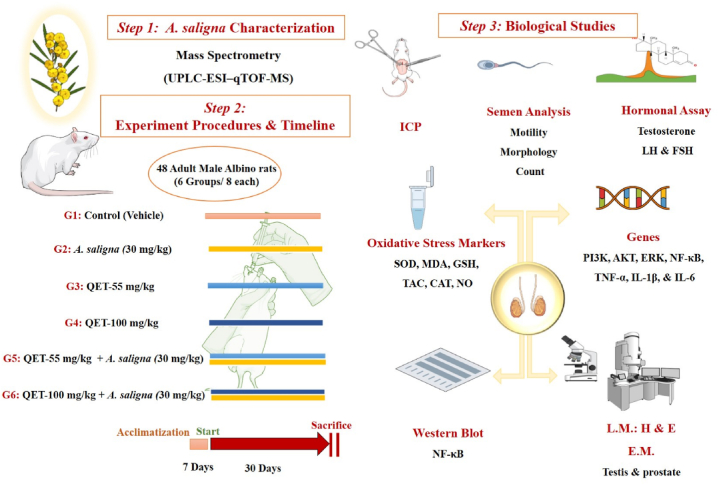
Rats were randomly assigned into six groups (8 rats/per group). The control group (G1) received a vehicle (1 mL/kg/day of normal saline, 0.9 % NaCl). The A. saligna group (G2) received A. saligna extract at 30 mg/kg [31]. The QET-55 group (G3) received a 55 mg/kg dose of QET, while the QET-100 group (G4) received a 100 mg/kg dose of QET [41]. The A. saligna + QET-55 group (G5) received A. saligna extract at 30 mg/kg 1 h after a 55 mg/kg dose of QET. Similarly, the A. saligna + QET-100 group received A. saligna extract at 30 mg/kg 1 h after a dose of QET at 100 mg/kg was administered. All interventions were given via oral gavage (o.g.) once daily for 30 days. At the end of the intervention period, rats were anesthetized for euthanasia by intraperitoneal injection (i.p.) of thiopental Na (40 mg/kg) [42]. For a summary of the methodological approach, refer to Fig. 1.
Fig. 1.
Diagrammatic sketch showing study design including A. saligna metabolites profiling for its flower alcoholic extract. After seven days of acclimatization, the animals were assigned into six groups, and the interventions were given by oral gavage once daily for 30 days. The groups included a control group, two QET groups (55 and 100 mg/kg), and two combined groups (QET + A. saligna). At the end of the experiment, a battery of investigations were done. Intracavernous pressure, semen analysis, and hormonal assays were done before animal sacrifice. Testicular tissue oxidative stress markers assays, inflammatory cytokines assays, gene and Western blot analyses, and histological assessment by light and electron microscopes were done after scarification.
2.6. Intracavernosal pressure (ICP)
After four weeks, all rodents' erectile responses were evaluated. The abdomen was cut open by a repeat midline abdominal incision, the cavernous nerves (CNs) were revealed and isolated, and the crus of the penis were determined. The maximum ICP, changes in ICP, the area under the ICP curve, and the ratio of change in ICP to mean arterial pressure were all monitored in real-time (Biopac Systems Inc., USA) [43]. For further details, refer to Supplement S1.
2.7. Semen analysis
Epididymides were dissected aseptically and filtered through 80 M pore size nylon-mesh onto sterile Petri plates containing 1 mL of pre-warmed (35 °C) Dulbecco's phosphate buffered saline (PBS) (CAT.NO. J67802.K2, Thermo Scientific, UK). The spermatozoa that were stained or partly stained were deemed dead. However, for sperm abnormalities examination, 100 spermatozoa (heads only or entire sperm) in each animal were microscopically examined for head/or flagellar problems. The anomalies in the head and tail were counted per 1000 sperm count [44]. For further details, refer to Supplement S2.
2.8. Estimation of oxidative stress and antioxidant biomarkers in testicular tissues
Superoxide dismutase enzyme (SOD) (CAT. No. SD 25 21), Malondialdehyde (MDA) (CAT. NO. MD 25 29), Glutathione (GSH) (CAT. NO. GR 25 11), Total antioxidant capacity (TAC) (CAT. NO. TA 25 13), Catalase (CAT) (CAT. NO. CA 25 17), and nitric oxide (NO) (CAT. NO 25 33) levels were assessed in testicular tissue homogenates. The results are expressed as (nmol/g for MDA), (mmol/g for GSH, and TAC), (U/g for SOD, and CAT) and (μmol/g for NO). All steps were carried out according to the manufacturer's protocol of the Biodiagnostic Company, Giza, Egypt.
2.9. Molecular RT-PCR analysis
Total RNA was obtained after homogenizing 20 mg of the testicular tissues (n = eight rats/group) using miRNeasy Mini Kit (CAT. NO.217004 QIAGEN GmbH, Germany). Quantitative analysis of targeted genes, including PI3K, AKT, ERK, NF-ҡB, TNF-α, IL-1β, and IL-6, was performed using step one real-time PCR instrument (CAT. NO. 4376357, Thermo Scientific, UK), for further details, refer to Supplement S3. The mRNA fold changes for the target genes were calculated using the 2-△△Ct approach, and gene expression was normalized to the expression of the β-actin gene, Table 1S.
Table 1.
Effect of QET and A. saligna on sperm parameters of rats.
|
Groups |
Sperm parameters |
||
|---|---|---|---|
| Sperm Count % (106/mL) |
Motility (%) |
Morphology Normal (%) |
|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| G1 Control | 80.143 ±0 .553 | 83.714 ± 1.409 | 82.143 ± 1.100 |
| G2 (A. salinga) | 79.857 ±0 .800 | 82.429 ± 1.251 | 81.429 ± 1.429 |
| G3 (QET-55) | 66.429 ± 1.307a,c | 72.571 ±0 .649a | 66.857 ± 1.503a,c |
| G4 (QET-100) | 41.571 ± 1.462a,b | 66.786 ± 1.011a | 54.714 ± 1.769a,b |
| G5 (A. saligna + QET-55) | 69.000 ± 1.826 | 81.571 ± 1.088b | 67.857 ± 2.064 |
| G6 (A. saligna + QET-100) | 44.571 ±0 .997 | 67.071 ± 1.441 | 59.143 ± 1.993 |
Data were analyzed using one-way ANOVA test. A statistically significant p-value was set at < 0.05.
: significant with the control group.
: significant with the G3.
: significant with the G4.
2.10. Western blot analysis
Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes and visualized by using diluted primary antibodies (cell signaling technology company, Danvers, MA, USA) (1:1000) for NF-κB p65 (CAT. NO. 8242) and β-actin (CAT. NO. 4970S, Cell Signaling company) followed by incubation with HRP-conjugated secondary antibodies. Protein detection was performed using enhanced chemiluminescence according to the manufacturer's instructions (ThermoFisher Scientific, United Kingdom) and quantified using the ChemiDoc imaging system (Bio-Rad, USA). For further details, refer to Supplement S4.
2.11. Histopathology examination
2.11.1. Hematoxylin and eosin staining (H&E)
The left testes and prostate glands were fixed in 10 % neutral formalin, embedded in paraffin, and then cut into five μm thick sections, stained with H&E stain, according to Bancroft and Layton[99]. The sections were examined using an Olympus Light Microscope to determine possible cytoarchitectural changes.
2.11.2. Periodic acid Schiff (PAS)
The left testes paraffin sections were cut into five μm thick sections to be stained by PAS according to Bancroft and Layton [99].
2.11.3. Morphometric study
Tubular epithelium thickness was measured through five H&E-stained slides from five different animals in each group and examined by Fiji Image J (1.51n; National Institute of Health; NIH, Bethesda, MD, USA).
2.11.4. transmission electron microscope
For electron microscopic analysis, the right testes from all experimental groups were fixed in 2.5 % glutaraldehyde solution in 0.1 M cacodylate buffer. Subsequently, they were postfixed in 1 % osmium tetroxide, dehydrated through a series of ethanol gradients, and embedded in epon Araldite. Using a LEICA Ultra microtome, 1-μm semithin sections were prepared, mounted on glass slides, and stained with toluidine blue. Ultra-thin sections were then cut, stained with 8 % uranyl acetate in 70 % ethanol, and further stained with lead citrate. The stained sections underwent analysis and photography using a Joel CX 100 transmission electron microscope, operated at an accelerating voltage of 60 kV, at the electron microscopic unit within the Faculty of Science, Zagazig University.
2.12. Statistical analysis
The statistical analysis of the data was performed using SPSS version 25 software. The data was expressed as mean values ± standard error of mean (SEM). Tukey's post hoc descriptive test and one-way ANOVA were used to test for significant differences between groups. A statistically significant p-value was set at < 0.05.
3. Results
3.1. Metabolites profiling of A. saligna extract via UPLC-MS/MS
By employing an untargeted UPLC-MS/MS method, we were able to accurately identify and classify 65 metabolites in the A. saligna extract. These metabolites belong to several chemical classes, including amino and phenolic acids, tannins, flavonoids, fatty acids, and sphingolipids. This comprehensive characterization serves as a helpful point of reference for future studies on A. saligna and its bioactive constituents, supporting its potential medicinal applications.
In the present study, an untargeted UPLC-MS/MS approach was employed to characterize metabolites in A. saligna alcohol extract. Gradient eluting using formic acid in water (0.1 %): acetonitrile allowed for the comprehensive elution of all metabolites within ca. 18 min, Fig. 1S. In total, 65 metabolites were annotated using positive and negative modes belonging to different chemical classes: amino and phenolic acids, tannins, flavonoids, fatty acids and their amides, and sphingolipids. Noteworthy, the negative ESI spectra allowed for better detection of phenolic acids, tannins, flavonoids, and fatty acids owing to the presence of phenyl and/or carboxylic groups, whereas the positive ion mode showed better performance for amino acids and fatty amides owing to the presence of nitrogen atoms that ionize better in that mode. The annotation of the detected metabolites was based on their retention times, experimental m/z, molecular formulas, mass errors, and MS2 fragments, as shown in Table 2S.
Table 2.
Oxidative and antioxidant biomarkers.
| Group Markers | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| SOD (U/g) | 2032 ± 77.8 | 2109.7 ± 127.8 | 1438.3 ± 47.9a,b | 1161 ± 104.5a,b | 2046.7 ± 68.5c | 1807 ± 67b,d |
| MDA (nmol/g) | 103.3 ± 3.5 | 105.3 ± 5.9 | 128 ± 2.6a,b | 134 ± 20.1a,b | 104.7 ± 4.7c | 119 ± 5.3a,c,d |
| GSH (mmol/g) | 31.7 ± 1.5 | 36 ± 2.6 | 21.3 ± 0.6a,b | 18.3 ± 1.5a,b | 44.6 ± 2.1a,b,c | 20.3 ± 1.5a,b,d |
| TAC (mmol/g) | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.1a,b | 0.3 ± 0.1a,b | 0.5 ± 0.1a | 0.4 ± 0.1a,b |
| CAT (U/g) | 22.5 ± 5 | 24.3 ± 1.5 | 11.5 ± 1.3a,b | 12.2 ± 0.3a,b | 24.2±1c | 21.3 ± 0.6b,d |
| NO (μmol/g) | 33.7 ± 0.9 | 36 ± 1 | 121.5 ± 1.3a,b | 138.7 ± 5.1a,b,c | 72.2 ± 10.1a,b,c | 124±1a,b,d,e |
CAT: Catalase, GSH: Glutathione, MDA: Malondialdehyde, NO: nitric oxide, SOD: Superoxide Dismutase enzyme, TAC: Total antioxidant capacity. Significant.
: compared to control (-ve).
: compared to control (+ve).
: compared to QET 55.
: compared to QET 100.
: compared to QET55+A. saligna. The data were analyzed using one-way ANOVA test. A statistically significant p-value was set at < 0.05.
3.1.1. Identification of amino and phenolic acids
Four amino acids were primarily characterized using positive ionization mode, including arginine (m/z 175.119, peak 1), asparagine (m/z 131.0469, peak 2), tyrosine (m/z 182.0814, peak 5), and tryptophan (m/z 205.0969, peak 12). These compounds showed a characteristic loss of 17 Da corresponding to the terminal amino moiety confirming their structure.
On the other hand, negative spectra aided in better detection of four phenolic acid hexosides such as gallic acid-O-hexoside (m/z 331.0677, peak 7), gentisoyl-hexoside (m/z 315.0742, peak 9), syringic acid-O-hexoside (m/z 359.0997, peak 11) and p-coumaric acid-O-hexoside (m/z 325.0944, peak 14). The identification of these metabolites was based on the loss of 162 Da corresponding to hexosyl moiety. Of all phenolic acid conjugates, only free gallic acid was detected with [M − H] − at m/z (169.0147, C7H5O5−), and MS/MS fragment ion at m/z 125 corresponded to the loss of its carboxyl moiety with a mass difference of 44 Da.
3.1.2. Identification of flavonoids
Several flavonoids were identified in A. Saligna extract based on their ESI-MS/MS spectra (Table 2S) agrees with the reported literature [29,45]. The identified flavonoids were free, glycosylated and/or acylated derivatives with characteristic MS/MS fragmentation patterns depending on the sugar type, e.g., hexose, pentose, rhamnose, acetyl hexose, and rutinoside, in addition to linking atom, i.e., O versus C-glycoside [46].
In detail, five quercetin glycosides were identified based on the characteristic product ion at m/z 303 [M+H]+ related to quercetin aglycon fragment such as peak 22 at m/z 611.1601 [M+H]+ annotated as quercetin-O-hexosyl rhamnoside (rutin) supported by product ions at m/z 465 [M + H−146]+for the loss of the rhamnosyl moiety and m/z 303 [M + H−146−162]+for further loss of a further hexosyl moiety. Moreover, the dereplication of quercetin-O-di-pentoside (peak 26), quercetin-O-pentoside (peak 31), and quercetin O-hexoside (peak 35) was based on the abundant aglycon fragment at m/z 303 due the loss of the di pentosyl (-2x132 Da), pentosyl (−132 Da) and hexosyl (−162 Da) moieties, respectively. Interestingly, a comparable fragmentation pattern was likewise detected for kaempferol aglycon and its glycosides such as peaks 28, 36, 41 and 43, corresponding to kaempferol-O-hexosyl rhamnoside, kaempferol-O-pentoside, kaempferol-O-hexoside, and kaempferol-O-rhamnoside, respectively, and also revealing the typical sugar losses of hexose (−162 Da), pentose (−132 Da) and rhamnose (−146 Da).
Methoxy flavonoids and their hexosides were likewise detected in A. saligna extracts such as patuletin-O-hexoside (peak 19), syringetin-O-hexoside (peak 32), hesperetin-O-hexoside (peak 40) and rhamnetin-O-hexoside (peak 42). These metabolites showed a corresponding loss of hexosyl moieties with MS/MS product ions of their aglycone fragments at m/z 333, 347, 301, and 317, respectively. Furthermore, peak 20 at m/z 771.2338 [M+H]+ was assigned as rhamnetin-O-hexosyl di -rhamnoside based on the successive loss of two rhamnosyl moieties at m/z 625 and 479, respectively, and the loss of the hexosyl moiety at m/z 317 yielding the aglycon fragment of rhamnetin. Likewise, peak 29 showed m/z 625.1757 [M+H]+, with MS2 fragment ions at 479 m/z [M + H-144]+ and m/z 317 [M + H−144−162]+ annotated as rhamnetin-O-hexosyl-rhamnoside.
Glycosides of myricetin, a hexahydroxyflavone with potential antioxidant activity [47], were detected in A. saligna extract represented by myricetin-O-pentoside (peak 25), myricetin-O-rhamnoside (peak 26), in addition to the acylated derivative myricetin-O-acetyl hexoside (peak 30). The latter's identification is based on the loss of the acetyl hexosyl moiety in the MS/MS spectra with a neutral loss of (204 Da), yielding the aglycon fragment of myricetin at m/z 319. Asides, two flavan-3-ol peaks were detected, including catechin-O-hexoside (peak 10) and (epi)catechin gallate (peak 27).
3.1.3. Identification of tannins and anthocyanins
Two B-type procyanidin isomers (peaks 13, 14) identified as procyanidin B1 were detected in the studied A.saligna extract. The peaks showed deprotonated and protonated molecular ions at m/z (577.1373, C30H25O12−) and m/z (579.1492, C30H27O12+), respectively, indicating a procyanidin dimer with B-type interflavanyl bonds and showing main MS/MS fragment ions at m/z 425 [M− H−152]− resulting from the Retro Diels–Alder fission and m/z 287 resulting from the quinine methide cleavage pathway typifying the common fragmentation pathway of type B procyanidins [48]. In addition, another anthocyanin was characterized in peak 39, with m/z at 449.107 and MS/MS fragment ions at m/z 317 identified as cyanidin-O-hexoside.
3.1.4. Identification of fatty acids and lipids
Many unsaturated and hydroxylated fatty acids were also characterized in A. saligna extract shows typical loss of H2O and/or loss of carboxylic moieties. For example, peak 56 with [M+H]+ at m/z 279.232 showed a fragment ion at m/z 261 corresponding to the loss of 18 Da and identified as octadecatrienoic acid. In addition, a mass difference of 16 amu between octadecatrienoic acid and peak 59 was indicative of an extra hydroxy group assigning peak 59 as a hydroxy derivative of octadecatrienoic acid. Likewise, peaks 49 (m/z 327.2182, C18H31O5−) and 50 (m/z 329.2339, C18H33O5−) were tentatively characterized as trihydroxy derivatives of octadecadienoic acid and octadecenoic acid, respectively, with two amu mass differences indicative for the extra double bonds. Both compounds showed MS/MS fragment ions at m/z 211 due to the C15\C16 bond cleavage.
Fatty acid amides were also detected in A. saligna extract via positive ionization mode as indicated from their even mass weights, suggesting the presence of nitrogen atom in lipids such as tetradecanamide (peak 61), palmitamide (peak 64), and oleamide (peak 65). Moreover, two sphingolipid peaks were also detected and annotated, namely, dehydrophytosphingosine (peak 54) at m/z 316.2837 and phytosphingosine (peak 55) at m/z 318.2991 with a mass difference of 2 amu indicative of the extra double bond.
3.2. Biological studies
A. saligna co-administration significantly enhanced sperm motility (p < 0.05), linked to its antioxidant and anti-inflammatory actions that protect cellular function and integrity. Although sperm count and morphological gains were not statistically significant, the trend toward normalization implies that an improved dose or prolonged treatment may be beneficial.
3.2.1. Effects on rat intracavernosal pressure (ICP)
Fig. 2-upper part shows images of ICP in response to cavernous nerve electrical stimulation. Fig. 2-lower part reveals that G4 (QET in a dose of 100 mg/kg) had a significantly decreased ICP (p < 0.05) compared to the control group, indicating deterioration of erectile function after QET intoxication. Additionally, G4 had significantly decreased ICP compared to G3 (dose-dependent effect). Co-administration of A. saligna with QET showed insignificant improvement in ICP compared to groups G3 and G4. All groups had no significant mean arterial blood pressure (MAP) difference (p > 0.05).
Fig. 2.
A. Response to cavernous nerve electrical stimulation. B. Mean ICP. a: significant with the control group.; b: significant with the G3. Data were analyzed using one-way ANOVA test. A statistically significant p-value was set at < 0.05.
3.2.2. A. saligna has a potential protective effect on sperm motility but not on count and morphology
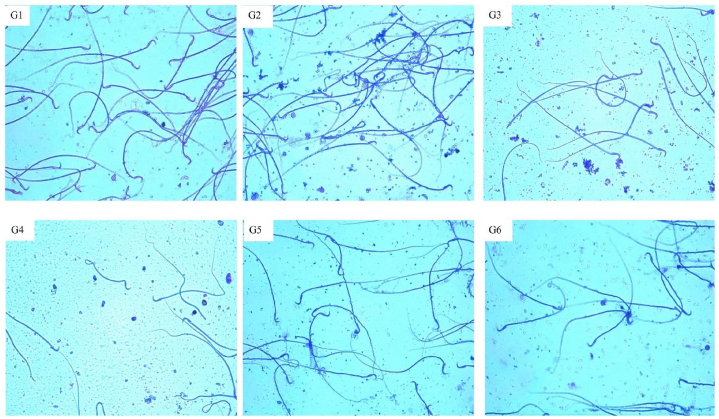
The rat sperm count, motility, and morphology indices showed insignificant difference among the control and A. saligna groups, G1 and G2 (p > 0.05). Compared with the control group, there was a statistically significant decrease (p < 0.05) in the index of sperm count, motility, and normal morphologic sperms in groups G3 and G4. Compared together, sperm count and normal sperms were diminished in both G3 and G4 in a dose-dependent manner. Co-administration of A. salinga with QET-55 showed a significantly increased (p < 0.05) sperm motility rate in comparison with G3. Co-administration of A. saligna with QET revealed insignificantly improved sperm count and morphology compared with groups G3 and G4, Table 1. Fig. 3, Fig. 4 demonstrate changes in rat sperm morphology. Therefore, A. saligna has protected against decreased sperm motility in QET-55 and not in QET-100; however, its effect on the morphology and count was insignificant with both doses of QET.
Fig. 3.
Photomicrographs of semen smears showing sperm morphology in the different experimental groups.
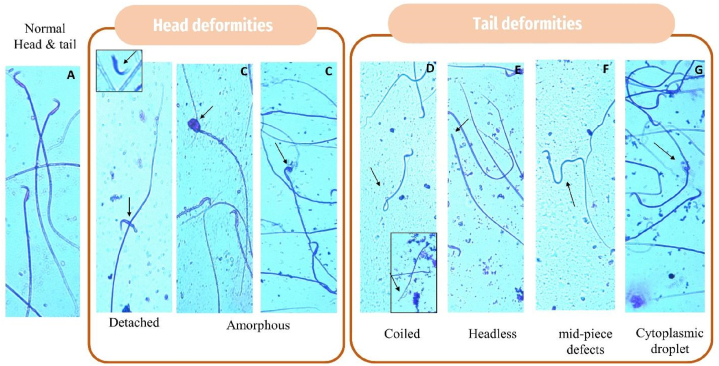
Fig. 4.
Photomicrograph of spermatozoa stained with 2 % eosin. A: normal sperm; B & C: Head deformities, B: Detached, C: amorphous; D-G Tail deformities: D: coiled, E: headless, F: mid-piece bent tail, G: cytoplasmic droplet. The (small black arrow) indicates an abnormality of spermatozoa.
3.3. Biochemical and genetic studies
The study found that QET treatment led to an increase in inflammatory biomarkers, including PI3K, AKT ERK, NF-κB, TNF-α, IL-1β, and IL-6. This was associated with decreased antioxidant enzyme levels and elevated oxidative stress markers. However, A. saligna significantly reversed these effects. The downregulation of the PI3K/AKT/ERK pathway by A. saligna reduced oxidative stress and inflammation, restoring antioxidant enzyme levels. NF-κB, a transcription factor, was upregulated in QET-treated groups, leading to higher inflammation and oxidative damage. A. saligna's anti-inflammatory properties and regulation of oxidative stress biomarkers were supported. A. saligna reduced testosterone, LH, and FSH levels in QET-treated groups, especially at QET-55. This hormonal stabilization is essential for spermatogenesis and testicular function, strengthening A. saligna's protection against QET-induced endocrine disruption.
3.3.1. Effect of A. saligna on the oxidative stress state and the levels of antioxidant enzymes in rats with QET testicular toxicity
Both QET-55 & 100 mg significantly reduced the antioxidant biomarkers (SOD, GSH, TAC, and Catalase) and increased the oxidative stress markers (MDA and nitric oxide marker). These biomarkers were significantly reversed, approaching normal levels when combining A.salignawith the QET in a dose-dependent manner, Table 2.
3.3.2. Effect of A. saligna on the testicular disrupted hormone levels caused by QET
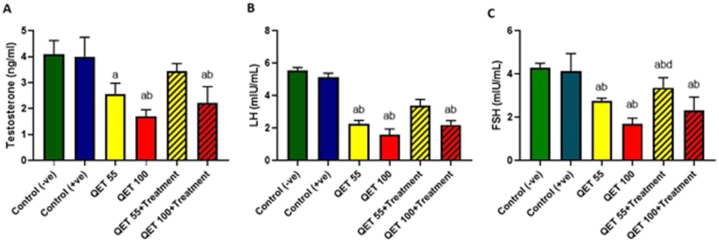
The study revealed that the administration of QET-55 and 100 mg resulted in a significant reduction of testosterone, LH, and FSH hormones. However, the co-administration of A.saligna with QET-55 demonstrated a protective effect, leading to a lesser reduction in hormone levels compared to the combination of A. saligna with QET-100 (dose-dependent protective effect), as shown in Fig. 5.
Fig. 5.
Hormonal levels in serum of different QET-treated groups compared to the controls; A. Testosterone, B. LH, and C. FSH.
Significant: a: compared to control (-ve), b: compared to control (+ve), c: compared to QET 55, d: compared to QET 100, e: compared to QET55+A. saligna. Data were analyzed using a one-way ANOVA test. A statistically significant p-value was set at < 0.05.
3.3.3. A. saligna mitigates the QET toxicity via NF-κB pathway and proinflammatory cytokines inhibition
The findings of our study indicate that QET toxicity induces a significant upregulation of various genes related to inflammatory pathways, including PI3K, AKT ERK, NF-κB, TNF-α, IL-1β, and IL-6. Furthermore, a comparison between the QET-55 A.saligna-treated and QET-100 A.saligna-treated groups revealed that A.saligna exerts a more pronounced effect in reducing the overexpression of inflammatory genes induced by QET-55. In contrast, no marked effect was observed in the case of QET-100 (suggesting a dose-dependent protective effect), as depicted in Fig. 6A–G.
Fig. 6.
Gene expression levels in testicular tissues of different A. saligna- QET-treated groups compared to the controls. A. PI3K gene expression, B. AKT gene expression, C. ERK gene expression, D. NF-κB gene expression, E. TNF gene expression, F. IL-1β gene expression, and G. IL-6 gene expression. Significant: a: Compared to control (-ve), b: Compared to control (+ve), c: Compared to QET 55, d: compared to QET 100, e: Compared to QET55+A. saligna.Data were analysed using one-way ANOVA test. A statistically significant p-value was set at < 0.05.
3.3.4. Effect of A. saligna on NF-κB protein expression level
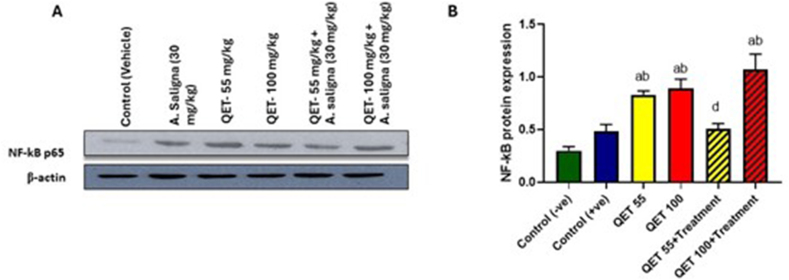
Our results showed that QET QET-55 and 100 mg significantly upregulate the NF-κB protein, as shown by the thicker bands in Fig. 3A, and the expression level of NF-κB was significantly prevented from being upregulated in QET-55 combined with A.saligna group with no marked effect of A. saligna on QET-100 treated groups (suggesting a dose-dependent protective effect), as displayed in Fig. 7-A and B.
Fig. 7.
Western blot analysis for the NF-κB protein expression on testicular tissues of different A. saligna- QET-treated groups. A. NF-κB protein expression versus the house keepinggene (β-actin) in the experimental groups and B. Statistical analysis of NF-κB protein expression in the experimental groups. a: Significant: a: compared to control (-ve), b: compared to control (+ve), c: QET 55 compared to QET 55+TTT, d: QET 55+TTT compared to QET 100+TTT. The data were analyzed using one-way ANOVA test. A statistically significant p-value was set at < 0.05.
3.4. Histological studies
The study found that A. saligna treatment improved histological integrity in testicular tissues, reducing oxidative stress and inflammatory infiltration. QET-treated groups had elevated MDA and nitric oxide levels, leading to seminiferous tubule disruption and decreased spermatogenesis. A. saligna reduced inflammatory cell infiltration and improved testicular morphology, suggesting it mitigates QET-induced inflammatory damage.
3.5. 1: H&E staining
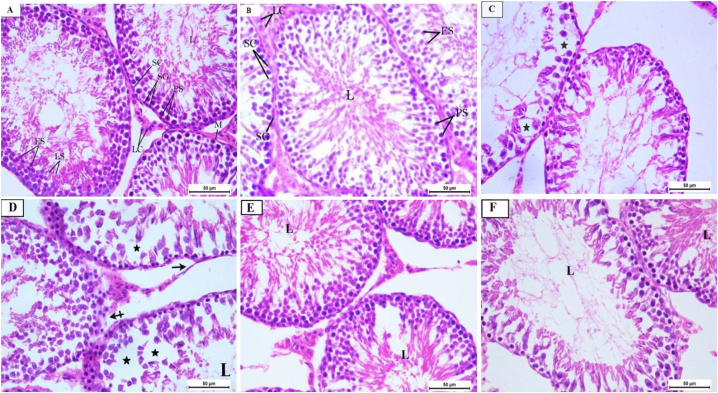
Histological examination of the testes indicates that QET toxicity induces tubular distortion, decreased spermatogenic cells, and areas of basement membrane loss in QET-55 with marked tubular deformation and atrophy of the germinal epithelium in QET-100. QET-55 and QET-100 A. saligna-treated groups revealed that A.saligna restored the typical tubular structure with areas of distorted germinal epithelium, and luminal spermatozoa loss is still noted in the higher dose, as depicted in Fig. 2, Fig. 8S.
Fig. 8.
(A–f): H&E photomicrographs sections of rat testes: G1 (A) and G2 (B) groups showing normal seminiferous tubules with Spermatogonia (SG) and Sertoli cells (SC) resting on the intact basement membrane, normal spermatogenic series; primary spermatocytes (PS), early spermatids (ES), late spermatids (LS), intact myoid cells (M) in the basement membrane and Leydig cells (LC) in the interstitial tissue and many sperms in the lumen (L). G3 (C) shows disorganization of the germinal epithelium of some seminiferous tubules and a decrease in the spermatogenic cells (star). G4 (D) shows marked disorganization of the seminiferous tubules, with sloughing of the germinal epithelium (star) and wide intercellular vacuolations, areas of detached basement membrane (black arrow) or lost basement membrane (dashed arrow), and decreased spermatozoa in the lumen (L). G5 (E) and G6 (F) groups show restoration of the normal organization of seminiferous tubules but in (F) with reduction of spermatozoa in the lumen (L). H&E X400.
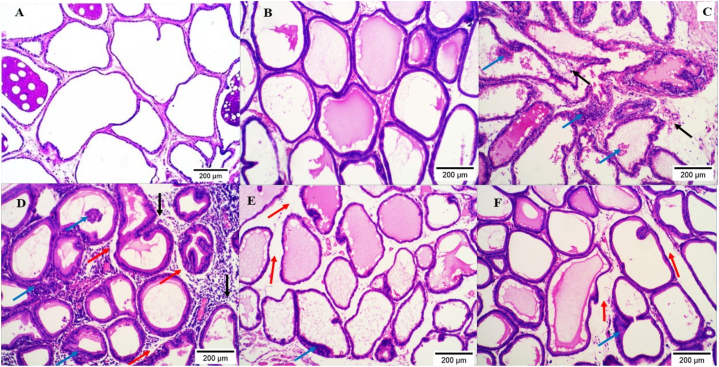
Histological examination of the prostate from our study groups indicates that QET-55 and QET-100 induce mild to severe prostatic inflammatory infiltration, interstitial edema, and desquamation of the glandular epithelium, respectively. QET-55 and QET-100 A. saligna-treated groups revealed marked improvement with minimal areas of epithelium desquamation in a dose-dependent protective manner, as shown in Fig. 9.
Fig. 9.
(A–f): H&E photomicrograph sections of rat prostate; G1 (A) and G2 (B) groups show normal and regularly arranged prostate glands. G3 (C) shows moderate focal inflammatory infiltration of peri glandular and perivascular tissue (black arrows) with areas of desquamation of the glandular epithelium (blue arrows). G4 (D) shows massive diffuse inflammatory infiltration in peri glandular, interstitial, and perivascular tissues (black arrows), interstitial edema (red arrows), and desquamation of the glandular epithelium (blue arrows). G5 (E) shows normal acini with interstitial edema (red arrow) and minimal desquamation of the glandular epithelium (blue arrow). G6 (F) shows a reduction of inflammatory infiltration with edema (red arrow) and desquamated glandular epithelium (blue arrow). H&E X100.
3.5.1. PAS staining
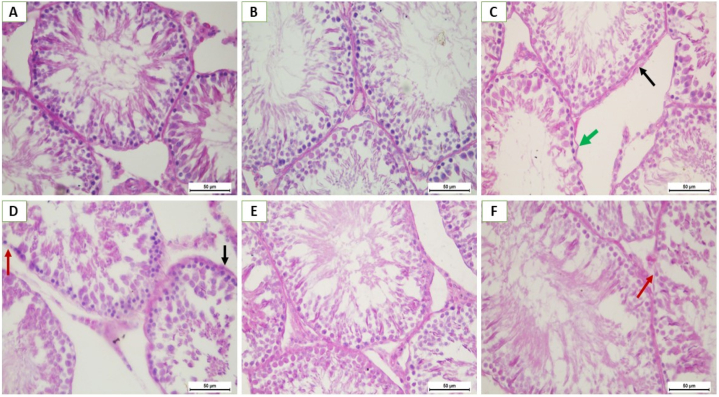
Histological examination of PAS-stained sections of the testes indicates that QET toxicity induces increased thickness and some irregularities of the stained basement membrane of the seminiferous tubules in QET-55 with marked loss of membrane reaction alternate with areas of reaction thickened in QET-100. QET-55 and QET-100 A. saligna-treated groups revealed that A. saligna restored the normal PAS stained reaction of the basement membrane with small areas of reaction loss in the QET-100 A. saligna-treated group, as displayed in Fig. 10.
Fig. 10.
(A–f): PAS- stain photomicrographs sections of rat testes: G1 (A) and G2 (B) groups showing normal germinal epithelium resting on regular, thin PAS-positive basement membrane. G3 (C) shows areas of thickened (black arrow) and irregular (green arrow) PAS-positive membrane. G4 (D) areas of thickened PAS-positive basement membrane (black arrow) and marked loss of membrane PAS reaction (red arrow). G5 (E) and G6 (F) groups show a thin, regular PAS-positive basement membrane of seminiferous tubules, but in (F), there are small areas of loss of membrane PAS reaction (red arrow). PAS X400.
3.5.2. Morphometric results
The current study revealed that administering QET-100 obviously reduced tubular epithelium thickness compared to the control, A. saligna, and QET-55- A. saligna-treated groups. QET-100 A. saligna-treated group showed a significant reduction compared to the control and A. saligna groups. Notably, QET-55+A. saligna administration caused a significant increase in tubular epithelium thickness compared to QET-100 and induced an increase compared to QET-55, but it was still insignificant. Fig. 3S.
3.5.3. Transmission electron microscope results
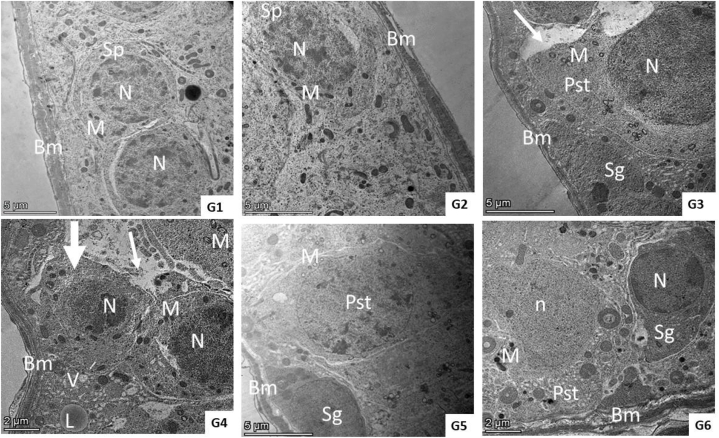
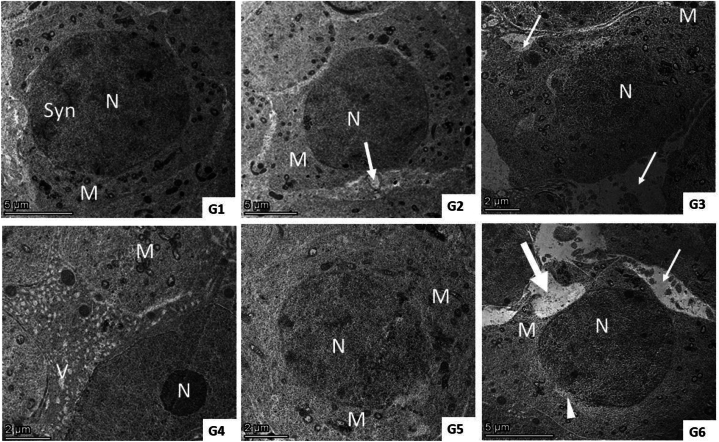
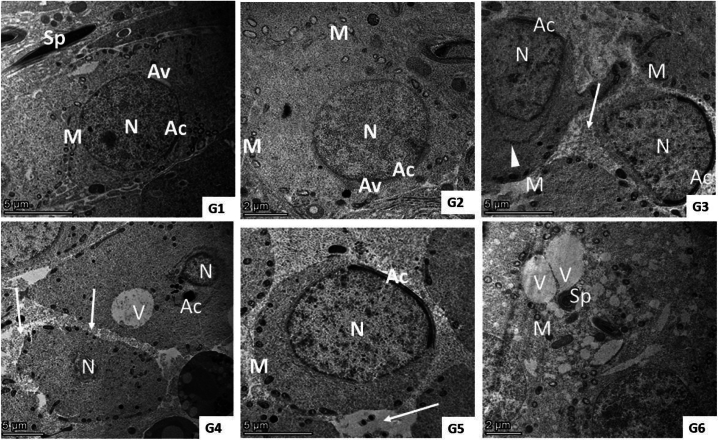
Ultrastructure examination of the QET-55 group showed distortion of seminiferous tubules ultrastructure; primary spermatocyte nuclear membrane damage, wide intercellular gap between a degenerated spermatocyte with irregular blebbing of its nucleus, spermatids with an irregular shrunken nucleus and irregular or detached acrosomal Cap. QET-100 showed more obvious ultrastructural damage in the form of shrunken spermatocyte, wide intercellular gap, lipid deposition and multiple vacuolations, necrotic spermatogonia, diffuse, marked necrosis of spermatid. QET-55 A. saligna-treated group revealed that A. saligna restored approximately normal seminiferous tubule with minimally enlarged primary spermatocytes having irregular nuclear outlines and numerous vacuolated mitochondria, normal spermatid. QET-55 A. saligna-treated group showed partial improvement of seminiferous tubular ultrastructure, some spermatocytes still having an irregularly shaped granular nucleus, spermatocytes with irregular cell outline with membrane damage, spermatids and spermatozoa having numerous cytoplasmic vacuolation and peripherally vacuolated mitochondria with irregularly shaped sperm, as depicted in Fig. 11, Fig. 12, Fig. 13.
Fig. 11.
(G1– G6): TEM photomicrograph of seminiferous tubular lining cells from different experimental groups. Groups (1and 2) showed normal basement membrane (Bm) surrounded by a thin connective tissue sheath containing myoid cells and lined with spermatogenic cells (SP) which had a normal nucleus (N) and normally arranged mitochondria (M). Group (3) showed a normal basement membrane (Bm), spermatogonia (Sg), primary spermatocyte (Pst) with nuclear membrane damage (N), granular cytoplasm, numerous vacuolated mitochondria (M), an irregular cell outline, and wide intercellular gap (arrow). Group (4) showed a normal basement membrane (Bm), shrunken spermatocyte (thick arrow) with a compact, granular electron-dense nucleus (N), granular cytoplasm, compact mitochondria (M) with minimal loss of cristae beside a wide intercellular gap (arrow), lipid deposition (L) and multiple vacuolation (V). Group (5) showed approximately normal seminiferous tubules having normal basement membrane (Bm), spermatogonia (Sg) with minimal enlarged primary spermatocytes (pst) having an irregular nuclear outline, and numerous vacuolated mitochondria (M). Group (6) showed a normal basement membrane (Bm), spermatogonia (sg), with normal nucleus (N), and spermatocyte (pst) with an irregularly shaped granular nucleus (n) and a few compact electron-dense mitochondria (M).
Fig. 12.
(G1– G6): TEM photomicrograph of primary spermatocytes from different experimental groups. G1) shows a primary spermatocyte with a prominent, well-defined euchromatic nucleus (N), synaptonemal complex (syn), granular cytoplasm, and many small mitochondria (M). G2) primary spermatocytes appear as controls with minimal loss of epithelial tight junctions (arrows). Group (3) shows loss of tight junctions (arrow) between a degenerated spermatocyte with an irregular blebbing nucleus (N) and few electron-dense mitochondria (M). Group (4) shows necrotic spermatogonia (N) and spermatocytes with complete loss of nucleus, numerous cytoplasmic vacuoles (V), and irregular vacuolated mitochondria with loss of cristae (M). Group (5) shows enlarged spermatocytes with dilated nuclei (N) and irregular, indefinite nuclear membranes, and dilated irregularly shaped mitochondria (M) with complete to few losses of cristae. Group (6) spermatocytes showed an irregular cell outline with membrane damage (thick arrow) and focal nuclear membrane damage (arrow head), numerous vacuolated mitochondria (M), and a wide intercellular gap (arrow).
Fig. 13.
(G1– G6): TEM photomicrograph of spermatids from different experimental groups. Groups (1 and 2) show normal spermatids with their characteristic incomplete acrosomal cap (AC) and acrosomal vesicles (AV). Its nucleus (N) is euchromatic with fine granular chromatin and peripherally arranged vesicular mitochondria (M) beside sperm in the lumen (Sp). Group (3) showed irregular cell outline with membrane rupture, loss of tight junction between cells (arrow), distribution of irregularly shaped (elongated to compact) mitochondria (M), irregular shrunken nucleus (N) with irregular (AC), and area of detached AC (arrow head). Group (4) shows diffuse, marked necrosis of spermatid with shrunken nucleus (N) and detached or loss of acrosomal cap (AC) besides large cytoplasmic vacuoles (V) and loss of tight junction (arrows). Group (5) shows normal spermatids with acrosomal caps (AC) on the nucleus (N) and peripherally arranged mitochondria (M) with minimal loss of tight junction between cells (arrow). Group (6) shows spermatids and spermatozoa having numerous cytoplasmic vacuolations (V) and peripherally vacuolated mitochondria (M) with irregularly shaped sperm (SP).
4. discussion
The current study aimed to assess the role of A. saligna flower extract in mitigating QET-induced sexual toxicity with two different doses of QET. A. saligna's characterization revealed its richness with a wide range of bioactive constituents, including phenols, flavonoids, fatty acids, and amino acids. Results revealed the dose-dependent effect of QET in inducing testicular toxicity. Moreover, this study unraveled the potential protective role of A. saligna in the lower dose of QET via functional, biochemical, genetic, and structural evidence. The NF-κB pathway, proinflammatory cytokines, and oxidative markers are shown to play a mechanistic role in the pathology and amelioration of sexual toxicity. The integrated genetic, biochemical, and histological data strongly support A. saligna's protection against QET-induced sexual toxicity. Downregulation of inflammatory pathways (PI3K, AKT ERK, NF-κB) and cytokines (TNF-α, IL-1β, IL-6) by A. saligna might lead to increased antioxidant status, reduced oxidative stress, and better histological outcomes. These data suggest that A. saligna may treat drug-induced reproductive toxicity.
4.1. Evidence of QET-induced sexual toxicity
Few studies focused on the effect of QET on sexual function. Nevertheless, some earlier studies have described the dose-dependent effects of quetiapine [49]. The pathophysiology behind the relationship between antipsychotic medications and sexual dysfunction remains unclear ([3,24] have found that tubular distortion and cytoplasmic vacuolation were more pronounced in rats administered QET-20 & 40 for 28 days compared to QET-10 [22]. Additionally, they observed that LH and testosterone levels decreased with both doses of QET. Moreover, catalase, SOD were diminished, while MDA was decreased with the high dose of QET ([22,50] have found no significant effects of QET- 10, 20, 40 for 21 days on the sexual act Zhang et al.2011[50,52] compared the effects of multiple antipsychotic drugs on sexual function in vivo and in silico. They reported that the QET-treated group had significantly lower sperm parameters, elevated GSH levels, and diminished SOD levels [52]. In a study where rats were exposed to chronic stress and treated with QET-20 mg/kg, serum IL-6 decreased upon treatment with QET [53]. Previous reports have documented that oxidative stress may influence sperm quality [54].
The germinal epithelium's ability to distinguish between normal sperm and oxidative stress also affects Leydig cell steroidogenesis [55]. In the present research, testicular oxidative stress caused by lowered SOD and CAT activities and elevated MDA levels after QET treatment may have decreased sperm concentration and exacerbated aberrant sperm morphology. Testicular oxidative stress may disturb tissue cells, causing structural alterations [56]. Pathologic findings were found in QET-administered testicular tissue by histology. Micro-vacuolation of the basal Sertoli cell cytoplasm following a 10 mg/kg-QET injection was a common early sign of morphologic insult preceding germ cell degeneration [57]. The QET-administrated group had higher MDA levels in testicular tissue, suggesting that QET or its toxic metabolites could pass the hematotesticular barrier and cause oxidative stress in the testes [58]. Low testosterone levels lowered testicular tissue antioxidant enzyme expression, causing oxidative stress [[58], [59], [60]]. Hence, decreasing testosterone levels may cause testicular tissue oxidative stress [61].
The QET groups had lower testosterone, FSH, and LH levels in the current research. The harmonious travel of neural and hormonal impulses requires neurotransmitters for neuroendocrine control of reproductive activities [62]. Thus, QET may lower LH and testosterone by altering central dopaminergic activity. Our data show that LH and testosterone levels affect sperm quality. The current and earlier investigations strongly suggest that QET is hazardous to male rats' sexual functioning.
4.2. A. saligna mitigated the QET-induced toxicity via the P13K/NF-κB pathway, proinflammatory cytokines inhibition, and antioxidant effects
The triggering of NF-κB by proinflammatory cytokines like interleukin 1 (IL-1) and tumor necrosis factor (TNF), as well as the contribution of NF-κB in the expression of other proinflammatory genes such as cytokines, chemokines, and adhesion molecules, have long been considered a prototypical proinflammatory signaling pathway [63]. In response to extracellular signals, the Akt pathway boosts survival and growth. By phosphorylating various intracellular proteins, activated Akt regulates downstream reactions such as cellular survival, growth, proliferation, migration, and angiogenesis. Akt controls pro-apoptotic proteins by direct phosphorylation. Thus, the interaction of Akt and NF-κB is crucial in establishing inflammation and developing apoptosis [64,65].
In the current study, all the investigated proinflammatory cytokines genes (IL-1β, IL-6, and TNF) were upregulated with the administration of both doses of QET. However, A. saligna mitigated this effect in the QET-50 more than in the QET-100 group. The latter findings reflect the mitigation of these inflammatory cytokines by A. saligna extract. The same was observed concerning the NF-κB pathway as tested by gene expression. The latter finding was confirmed by assessing the NF-κB protein expression via Western blot analysis.
A. saligna is a rich source of a wide variety of bioactive compounds, including phenolics and flavonoids. These phenolics and flavonoids were described as potent inhibitory effects on inflammation [66]. According to previous studies, the anti-inflammatory effects of gallic acid were mainly mediated through the NF-κB and mitogen-activated protein kinases (MAPK) signaling pathways. Therefore, it reduces the inflammatory response by suppressing the production of chemokines, adhesion molecules, inflammatory cytokines, and cell infiltration [67]. p-Coumaric acid-O-hexoside was documented to have the ability to inhibit enzyme activity related to oxidative stress-induced erectile dysfunction and might be employed as an aphrodisiac medication in the management of erectile dysfunction [68]. The role of oxidative stress in controlling vascular erectile dysfunction has been investigated. Quercetin, a well-known antioxidant flavonoid, and its derivatives were stated to exhibit anti-inflammatory effect via regulation of nitric oxide synthase (eNOS) expression and activity in cavernous endothelial cells and to sustain normal function of the nitric oxide-cGMP pathway during penile erection [69]. An earlier study reported that kaempferol remarkably prevented the development of testosterone-induced benign prostatic hyperplasia [70]. Additionally, apigenin was reported to have the protection capability against ROS-induced testicular dysfunctions [71], the pro-inflammatory cytokines (IL-6, TNF-α and TGF 1-β) and oxidant enzymes (iNOS) along with its abilities to enhance the antioxidant enzymes (SOD, CAT, and GSH) [72]. Noteworthy, other researchers reported that apigenin has led to negative consequences for male mice's reproductive tracts [73]. Intriguingly, an earlier study reported that the sole use of l-arginine showed an adverse effect on male sexual function. However, quercetin prevented this adverse effect when combined with l-arginine [93]. A. saligna extract analysis revealed the presence of both substances and showed a beneficial effect on sexual function and structure. The aforementioned sheds light on the importance of a plant-based diet in managing sexual function due to the naturally occurring balanced bioactive substances. Many other studies have reported the protective role of quercetin on male sexual function [74,75] and structure [76]. According to studies, quercetin decreases testicular toxicity by reducing the generation of reactive oxygen species. Quercetin's radical-scavenging activity may affect the signal transduction of oxidative stress-induced apoptosis, decrease inflammation, and increase sperm quality [74]. In addition, quercetin was reported to decrease the levels of IL-1, IL-6, and TNF-α, which were elevated in diabetic rats [77]. Another major flavonoid, kaempferol (peaks 28, 36, 41, and 43), is reported to prevent the development of testosterone-induced benign prostatic hyperplasia [70].
The results of this study match prior research that supports the positive impacts of bioactive components found in A. saligna. Furthermore, these findings contribute to understanding how these components can protect the reproductive capabilities of male rats. Moreover, it can be observed that the fundamental mode of action is facilitated through the antioxidant and anti-inflammatory attributes of phenolics and flavonoids that are abundant in the extract, Fig. 14.
Fig. 14.
Suggested action mechanisms involved in the mitigation of QET-induced sexual toxicity. Both QET doses provoked the ROS activity that stimulated the PI3K– NF-κB pathway. The latter, in turn, stimulates the release of proinflammatory cytokines (IL-1β, IL-6, and TNF-α). The latter, in turn, disturbs the sexual structure and function. The PI3K– NF-κB pathway also stimulates oxidative stress, further affecting sexual structure and function. QET could directly provoke the PI3K– NF-κB pathway as well. On the other hand, A. saligna, via its bioactive constituents' antioxidant and anti-inflammatory properties, can counteract the mechanisms mentioned above that are involved in QET-induced sexual toxicity.
4.3. The suggested integrated role of A. salignaon mitigating sexual toxicity
The study revealed a notable increase in the expression of inflammatory biomarkers (PI3K, AKT ERK, NF-κB, TNF-α, IL-1β, and IL-6) in the groups treated with QET. This rise in expression was found to be associated with unfavorable biochemical alterations, such as decreased levels of antioxidant enzymes (SOD, GSH, TAC, and Catalase) and elevated levels of oxidative stress markers (MDA and nitric oxide). The combined intake of A. saligna significantly reversed these effects.
PI3K/AKT/ERK pathway activation is recognized for its role in controlling cell survival, proliferation, and inflammation [23]. The activation of this system generated by QET probably played a role in the increase of oxidative stress and inflammatory responses, as seen by elevated levels of MDA and nitric oxide. The downregulation of these pathways by A. saligna indicates a decrease in oxidative stress and inflammation, leading to the restoration of antioxidant enzyme levels to normal. NF-κB regulates inflammatory cytokine expression as a crucial transcription factor. Upregulation of NF-κB and downstream cytokines (TNF-α, IL-1β, IL-6) [34] in QET-treated groups led to higher inflammation and oxidative damage. The lowering of NF-κB and cytokine levels after A. saligna treatment supports its anti-inflammatory properties and regulation of oxidative stress biomarkers, Fig. 14. Histological investigation showed that QET caused substantial testicular damage, including tubular deformation, decreased spermatogenic cells, basement membrane loss, and prostatic damage, including inflammatory infiltration, interstitial edema, and glandular epithelium desquamation. These morphological changes match scientific findings because QET causes oxidative stress and inflammation, which damages tissues. Normalized tubular structure and preserved prostatic histology were seen after co-administration of A. saligna, which lowered inflammatory indicators and restored antioxidant levels.
A. saligna therapy reduced QET-induced oxidative stress and inflammatory infiltration, conserved seminiferous tubule structure and spermatogenesis, and decreased MDA and nitric oxide levels. A. saligna did not significantly improve ICP. This shows that A. saligna protects against QET-induced sexual toxicity through molecular, biochemical, histological, and functional improvements.
5. Conclusion
In conclusion, our study provides unprecedented insights into the promising protective effects of A. saligna against quetiapine (QET)-induced sexual toxicity in male rats. The observed downregulation of the NF-κB pathway, reduction in proinflammatory cytokines (IL-1β, IL-6, and TNF), and antioxidant properties underscore the multifaceted mechanisms underlying A. saligna's efficacy. Significantly, A. saligna's protective action, with heightened efficacy observed in the QET-55 group across functional and structural parameters, underscores its potential clinical relevance. Moreover, the synergy among multiple active constituents in A. saligna, mitigating the potential adverse effects of individual components, exemplifies its holistic therapeutic potential. However, the determination of precise effective doses and treatment durations for each bioactive constituent remains an avenue for future investigation. Continued research is warranted to elucidate A. saligna full spectrum of benefits and optimize therapeutic parameters for conclusive clinical applications. Further research is required to demonstrate the effects of the extract's active metabolites, and this includes standardizing and testing individual components.
Funding
The authors funded all the research project.
CRediT authorship contribution statement
Shimaa Mohammad Yousof: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Conceptualization. Shaimaa A. Shehata: Writing – original draft, Software, Resources, Project administration, Methodology, Investigation, Data curation, Conceptualization. Ezzat A. Ismail: Writing – original draft, Methodology, Investigation, Data curation. Samar M. Abd El-moneam: Writing – review & editing, Writing – original draft, Software, Resources, Methodology, Investigation. Basma S.A. Mansour: Writing – review & editing, Writing – original draft, Software, Resources, Methodology, Investigation. Mohamed A. Farag: Writing – review & editing, Validation, Resources, Methodology, Investigation. Abdelsamed I. Elshamy: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Abd El-Nasser G. El Gendy: Writing – original draft, Resources, Methodology, Investigation. Ahmed Serag: Writing – original draft, Resources, Methodology, Investigation. Noha M. Abd El-Fadeal: Writing – original draft, Visualization, Software, Resources, Methodology, Investigation, Data curation. Rehab Ibrahim Abdel-Karim: Writing – review & editing, Methodology, Investigation. Mostafa M. Mostafa: Writing – review & editing, Resources, Methodology. Dina H. Ibrahim: Writing – review & editing, Resources, Methodology. Mohamed A. Zayed: Writing – original draft, Resources, Methodology.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT 3.5 and Quillbot in order to paraphrase, correct language and to do grammar check. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Declaration of competing interest
No conflict of interest.
Acknowledgment
Nil.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e33993.
Contributor Information
Shimaa Mohammad Yousof, Email: smabrahem@kau.edu.sa.
Shaimaa A. Shehata, Email: shaimaa_shehata@med.suez.edu.eg.
Ezzat A. Ismail, Email: boezzat@med.suez.edu.eg.
Samar M. Abd El-moneam, Email: samar_mohamed@med.suez.edu.eg.
Basma S.A. Mansour, Email: basmasaid@med.suez.edu.eg.
Mohamed A. Farag, Email: mohamed.farag@pharma.cu.edu.eg.
Abdelsamed I. Elshamy, Email: elshamynrc@yahoo.com.
Abd El-Nasser G. El Gendy, Email: aggundy_5@yahoo.com.
Ahmed Serag, Email: ahmedserag777@hotmail.com.
Noha M. Abd El-Fadeal, Email: noha_abdelfadeal@med.suez.edu.eg.
Rehab Ibrahim Abdel-Karim, Email: rehab_ali@med.suez.edu.eg.
Mostafa M. Mostafa, Email: mmostafa@kau.edu.sa.
Dina H. El-Sheikh, Email: d.elsheikh@psau.edu.sa.
Mohamed A. Zayed, Email: mazayed@kau.edu.sa.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Organization W.H. 2020. WHO Global Meeting to Accelerate Progress on SDG Target 3.4 on Noncommunicable Diseases and Mental Health. 9–12 December 2019, Muscat, Oman: meeting report. [DOI] [PubMed] [Google Scholar]
- 2.Correll C.U., Detraux J., De Lepeleire J., De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatr.: Official Journal of the World Psychiatric Association (WPA) 2015;14(2):119–136. doi: 10.1002/wps.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Berardis D., Rapini G., Olivieri L., Di Nicola D., Tomasetti C., Valchera A., Fornaro M., Di Fabio F., Perna G., Di Nicola M., Serafini G., Carano A., Pompili M., Vellante F., Orsolini L., Martinotti G., Di Giannantonio M. Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine. Therapeutic Advances in Drug Safety. 2018;9(5):237–256. doi: 10.1177/2042098618756261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samplaski M.K., Nangia A.K. Adverse effects of common medications on male fertility. Nat. Rev. Urol. 2015;12:401–413. doi: 10.1038/nrurol.2015.145. [DOI] [PubMed] [Google Scholar]
- 5.Drobnis E.Z., Nangia A.K. Impacts of Medications on Male Fertility. 2017. Psychotropics and male reproduction; pp. 63–101. [DOI] [PubMed] [Google Scholar]
- 6.Semet M., Paci M., Saïas‐Magnan J., Metzler‐Guillemain C., Boissier R., Lejeune H., Perrin J. The impact of drugs on male fertility: a review. Andrology. 2017;5:640–663. doi: 10.1111/andr.12366. [DOI] [PubMed] [Google Scholar]
- 7.Solomon R., Shvartsur R., Azab A.N. The association between psychotropic drug use and fertility problems among male subjects. Journal of Psychiatric Practice®. 2019;25:22–33. doi: 10.1097/PRA.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari P., Panik R., Bhattacharya A., Ahirwar D., Chandy A. Evidences of possible side effects of neuroleptic drugs: a systematic review. Asian Pacific Journal of Reproduction. 2012;1:330–336. [Google Scholar]
- 9.Gao K., Sheehan D.V., Calabrese J.R. Atypical antipsychotics in primary generalized anxiety disorder or comorbid with mood disorders. Expert Rev. Neurother. 2009;9:1147–1158. doi: 10.1586/ern.09.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poyurovsky M., Braverman L., Weizman A. Beneficial effect of QET monotherapy in patients with bipolar depression and comorbid obsessive-compulsive disorder. Int. Clin. Psychopharmacol. 2020;36:50–53. doi: 10.1097/YIC.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 11.Vilibić M., Peitl V., Živković M., Vlatković S., Ljubičić Bistrović I., Ljubičić R., Matošić A., Karlović D. QET add-on therapy may improve persistent sleep disturbances in patients with PTSD on stabile combined SSRI and benzodiazepine combination: a one-group pretest-posttest study. Psychiatr. Danub. 2022;34:245–252. doi: 10.24869/psyd.2022.245. [DOI] [PubMed] [Google Scholar]
- 12.Monahan K., Cuzens-Sutton J., Siskind D., Kisely S. QET withdrawal: a systematic review. Aust. N. Z. J. Psychiatr. 2021;55(8):772–783. doi: 10.1177/0004867420965693. [DOI] [PubMed] [Google Scholar]
- 13.Jahnsen J.A., Widnes S.F., Schjott J. QET, misuse and dependency: a case-series of questions to a Norwegian network of drug information centers. Drug Healthc. Patient Saf. 2021;13:151–157. doi: 10.2147/DHPS.S296515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattson M.E., Albright V.A., Yoon J., Council C.L. Emergency department visits involving misuse and abuse of the antipsychotic QET: results from the Drug Abuse Warning Network (DAWN) Subst. Abuse Res. Treat. 2015;9 doi: 10.4137/SART.S22233. SART. S22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heilbronn C., Lloyd B., Mcelwee P., Eade A., Lubman D.I. Trends in QET use and non‐fatal QET‐related ambulance attendances. Drug Alcohol Rev. 2013;32:405–411. doi: 10.1111/dar.12028. [DOI] [PubMed] [Google Scholar]
- 16.Fountain J.S., Slaughter R.J. TOXINZ, the New Zealand Internet poisons information database: the first decade. Emerg. Med. Australasia (EMA) 2016;28:335–340. doi: 10.1111/1742-6723.12594. [DOI] [PubMed] [Google Scholar]
- 17.Jaehne E.J., Corrigan F., Toben C., Jawahar M.C., Baune B.T. The effect of the antipsychotic drug quetiapine and its metabolite norquetiapine on acute inflammation, memory and anhedonia. Pharmacol. Biochem. Behav. 2015;135:136–144. doi: 10.1016/j.pbb.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 18.AL-Amin M.M., Nasir Uddin M.M., Mahmud Reza H. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11:144–151. doi: 10.9758/cpn.2013.11.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baune B.T., Eyre H. Anti-inflammatory effects of antidepressant and atypical antipsychotic medication for the treatment of major depression and comorbid arthritis: a case report. J. Med. Case Rep. 2010;4:6. doi: 10.1186/1752-1947-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuo C., Xu Y., Hou W., Chen J., Li Q., Liu Z., Dou G., Sun Y., Li R., Ma X., Tian H., Zhou C. Mechanistic/mammalian target of rapamycin and side effects of antipsychotics: insights into mechanisms and implications for therapy. Transl. Psychiatry. 2022;12:13. doi: 10.1038/s41398-021-01778-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Peng Z., Zhou Y., Wang J., Lin X., Dong X., Liu X., Jiang J., Jiang Y., Li L. Quetiapine induces myocardial necroptotic cell death through bidirectional regulation of cannabinoid receptors. Toxicol. Lett. 2019;313:77–90. doi: 10.1016/j.toxlet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Korkut Celikates B., Kilic V., Atli-Eklioglu O. vol. 45. 2022. pp. 2379–2387. (Effects of QET Administration on Sperm Quality and Testicular Histology). [DOI] [PubMed] [Google Scholar]
- 23.He M., Fan J., Zhou R., Gao G., Li R., Zuo Y., Li B., Li Y., Sun T. NLRP3/Caspase-1-Mediated pyroptosis of astrocytes induced by antipsychotics is inhibited by a histamine H1 receptor-selective agonist. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.847561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korkut Celikates B., Kilic V., Atli-Eklioglu O., Baysal M., Aydogan-Kılıc G., Ucarcan S., Ilgin S. Effects of QET administration on sperm quality and testicular histology. Drug Chem. Toxicol. 2021;0(0):1–9. doi: 10.1080/01480545.2021.1946558. [DOI] [PubMed] [Google Scholar]
- 25.Yousof S.M., Awad Y.M., Mostafa E.M.A., Hosny M.M., Anwar M.M., Eldesouki R.E., Badawy A.E. The potential neuroprotective role of Amphora coffeaeformis algae against monosodium glutamate-induced neurotoxicity in adult albino rats. Food Funct. 2021;12:706–716. doi: 10.1039/d0fo01957g. [DOI] [PubMed] [Google Scholar]
- 26.Goyal S., Gupta N., Chatterjee S., Nimesh S. Natural plant extracts as potential therapeutic agents for the treatment of cancer. Curr. Top. Med. Chem. 2017;17(2):96–106. doi: 10.2174/1568026616666160530154407. [DOI] [PubMed] [Google Scholar]
- 27.Abdallah H.M.I., Ammar N.M., Abdelhameed M.F., Gendy A.E.-N.G.E., Ragab T.I.M., Abd-ElGawad A.M., Farag M.A., Alwahibi M.S., Elshamy A.I. Protective mechanism ofA.Saligna butanol extract and its nano-formulations against ulcerative colitis in rats as revealed via biochemical and metabolomic assays. Biology. 2020;9(8):E195. doi: 10.3390/biology9080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Midgley S., Turnbull J. Domestication and use of Australian A.salignas: case studies of five important species. Aust. Syst. Bot. 2003;16:89–102. [Google Scholar]
- 29.Elansary H.O., Szopa A., Kubica P., Ekiert H., A. AL-Mana F., AL-Yafrsi M.A. Antioxidant and biological activities of A.Saligna and lawsonia inermis natural populations. Plants. 2020;9:908. doi: 10.3390/plants9070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Huqail A.A., Behiry S.I., Salem M.Z.M., Ali H.M., Siddiqui M.H., Salem A.Z.M. Antifungal, antibacterial, and antioxidant activities of A.Saligna (labill.) H. L. Wendl. Flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules. 2019;24(4):700. doi: 10.3390/molecules24040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EL-Toumy S. 2006. Flavonoids from Acacia Saligna Leaves and Evaluation of Antihyperglycaemic Effect of Aqueous Extract. [Google Scholar]
- 32.Fahmy W., Anwar M., EL M., Sallam S., Attia M., Nour EL-Din A., Hashem N., EL Z.-S., Zeitoun M., Wakeel-Saeed A., EL . vol. 2. 2017. (Productive and Reproductive Traits of Sheep Fed Acacia Saligna Leaves-Based Diets). [Google Scholar]
- 33.Ibrahim Yousef M. Reproductive performance, blood testosterone, lipid peroxidation and seminal plasma biochemistry of rabbits as affected by feeding Acacia Saligna under subtropical conditions. Food Chem. Toxicol. : an international journal published for the British Industrial Biological Research Association. 2005;43:333–339. doi: 10.1016/j.fct.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Guo Q., Jin Y., Chen X., Ye X., Shen X., Lin M., Zeng C., Zhou T., Zhang J. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. 2024;9:53. doi: 10.1038/s41392-024-01757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman A., Fazal F. Blocking NF-κB: an inflammatory issue. Proc. Am. Thorac. Soc. 2011;8:497–503. doi: 10.1513/pats.201101-009MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai D., Ueno L., Vogt P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer. 2009;125:2863–2870. doi: 10.1002/ijc.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Confalone E., D'Alessio G., Furia A. IL-6 induction by TNFα and IL-1β in an osteoblast-like cell line. Int. J. Biomed. Sci. 2010;6:135–140. [PMC free article] [PubMed] [Google Scholar]
- 38.Ung A.T., Asmara A.P. Bioactive phytochemicals of Acacia saligna. Molecules. 2023;28:4396. doi: 10.3390/molecules28114396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khaled S.E., Hashem F.A., Shabana M.H., Hammam A.M., Madboli A.N.A., AL-Mahdy D.A., Farag M.A. A biochemometric approach for the assessment of Phyllanthus emblica female fertility effects as determined via UPLC-ESI-qTOF-MS and GC-MS. Food Funct. 2019;10:4620–4635. doi: 10.1039/c9fo00767a. [DOI] [PubMed] [Google Scholar]
- 40.Kabbash E.M., Abdel-Shakour Z.T., EL-Ahmady S.H., Wink M., Ayoub I.M. Comparative metabolic profiling of olive leaf extracts from twelve different cultivars collected in both fruiting and flowering seasons. Sci. Rep. 2023;13:612. doi: 10.1038/s41598-022-27119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh K.P., Tripathi N. Prenatal exposure to a novel antipsychotic QET: impact on neuro-architecture, apoptotic neurodegeneration in fetal hippocampus and cognitive impairment in young rats. Int. J. Dev. Neurosci. 2015;42:59–67. doi: 10.1016/j.ijdevneu.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Fish R., Danneman P.J., Brown M., Karas A. Academic press; 2011. Anesthesia and Analgesia in Laboratory Animals. [Google Scholar]
- 43.Liao C.H., Wu Y.N., Lin Y.H., Syu Huang R.F., Liu S.P., Chiang H.S. Restoration of erectile function with intracavernous injections of endothelial progenitor cells after bilateral cavernous nerve injury in rats. Andrology. 2015;3:924–932. doi: 10.1111/andr.12085. [DOI] [PubMed] [Google Scholar]
- 44.Greish S.M., Abdel Kader G.S., Abdelaziz E.Z., Eltamany D.A., Sallam H.S., Abogresha N.M. Lycopene is superior to moringa in improving fertility markers in diet-induced obesity male rats. Saudi J. Biol. Sci. 2021;28:2956–2963. doi: 10.1016/j.sjbs.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seigler D.S. Phytochemistry of A.saligna—sensu lato. Biochem. Systemat. Ecol. 2003;31:845–873. [Google Scholar]
- 46.Serag A., Baky M.H., Döll S., Farag M.A. UHPLC-MS metabolome based classification of umbelliferous fruit taxa: a prospect for phyto-equivalency of its different accessions and in response to roasting. RSC Adv. 2020;10:76–85. doi: 10.1039/c9ra07841j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ijaz M.U., Anwar H., Iqbal S., Ismail H., Ashraf A., Mustafa S., Samad A. Protective effect of myricetin on nonylphenol-induced testicular toxicity: biochemical, steroidogenic, hormonal, spermatogenic, and histological-based evidences. Environ. Sci. Pollut. Control Ser. 2021;28:22742–22757. doi: 10.1007/s11356-020-12296-5. [DOI] [PubMed] [Google Scholar]
- 48.Rue E.A., Rush M.D., Van Breemen R.B. Procyanidins: a comprehensive review encompassing structure elucidation via mass spectrometry. Phytochemistry Rev. 2018;17:1–16. doi: 10.1007/s11101-017-9507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt H.M., Hagen M., Kriston L., Soares-Weiser K., Maayan N., Berner M.M. Management of sexual dysfunction due to antipsychotic drug therapy. Cochrane Database Syst. Rev. 2012;11 doi: 10.1002/14651858.CD003546.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X.R., Zhang Z.J., Jenkins T.A., Cheng W.R., Reynolds G.P. The dose-dependent effect of chronic administration of haloperidol, risperidone, and QET on sexual behavior in the male rat. J. Sex. Med. 2011;8:3345–3353. doi: 10.1111/j.1743-6109.2010.01740.x. [DOI] [PubMed] [Google Scholar]
- 52.Dincer B., Bulent Yazici A., Cinar I., Toktay E., Selli J., Cadirci E., Bayraktutan Z., Yazici E. Antipsychotics induced reproductive toxicity by stimulating oxidative stress: a comparative in vivo and in Silico study. Chem. Biodivers. 2023 doi: 10.1002/cbdv.202201190. [DOI] [PubMed] [Google Scholar]
- 53.Grolli R.E., Bertollo A.G., Behenck J.P., de Araujo Borba L., Plissari M.E., Soares S.J.B., Manica A., da Silva Joaquim L., Petronilho F., Quevedo J., Bagatini M.D., Réus G.Z., Ignácio Z.M. Quetiapine effect on depressive-like behaviors, oxidative balance, and inflammation in serum of rats submitted to chronic stress. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(7):1423–1433. doi: 10.1007/s00210-023-02406-8. [DOI] [PubMed] [Google Scholar]
- 54.Dutta S., Majzoub A., Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab journal of urology. 2019;17:87–97. doi: 10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sedha S., Kumar S., Shukla S. Role of oxidative stress in male reproductive dysfunctions with reference to phthalate compounds. Urol. J. 2015;12:2304–2316. [PubMed] [Google Scholar]
- 56.Asadi N., Bahmani M., Kheradmand A., Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J. Clin. Diagn. Res.: J. Clin. Diagn. Res. 2017;11 doi: 10.7860/JCDR/2017/23927.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Creasy D.M. Pathogenesis of male reproductive toxicity. Toxicol. Pathol. 2001;29:64–76. doi: 10.1080/019262301301418865. [DOI] [PubMed] [Google Scholar]
- 58.Banerjee S., Ghosh J., Sil P. Drug metabolism and oxidative stress: cellular mechanism and new therapeutic insights. Biochem. Anal. Biochem. 2016;5:2161. 1009. [Google Scholar]
- 59.Ghosh D., Das U., Ghosh S., Mallick M., Debnath J. Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: a correlative study with testicular oxidative stress. Drug and chemical toxicology. 2002;25:281–292. doi: 10.1081/dct-120005891. [DOI] [PubMed] [Google Scholar]
- 60.Amaral S., Amaral A., Ramalho-Santos J. Aging and male reproductive function: a mitochondrial perspective. Frontiers in Bioscience-Scholar. 2013;5:181–197. doi: 10.2741/s365. [DOI] [PubMed] [Google Scholar]
- 61.Zini A., Bielecki R., Phang D., Zenzes M.T. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil. Steril. 2001;75:674–677. doi: 10.1016/s0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 62.Klett D., Cahoreau C., Villeret M., Combarnous Y. Effect of pharmaceutical potential endocrine disruptor compounds on protein disulfide isomerase reductase activity using di-eosin-oxidized-glutathion. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang B., Tang F., Wang Z., Qi G., Liang X., Li B., Yuan S., Liu J., Yu S., He S. Upregulation of Akt/NF-κB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: suppression by carnosic acid nanoparticle. Int J Nanomedicine. 2016;11:6401–6420. doi: 10.2147/IJN.S101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chagpar R.B., Links P.H., Pastor M.C., Furber L.A., Hawrysh A.D., Chamberlain M.D., Anderson D.H. Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2010;107:5471–5476. doi: 10.1073/pnas.0908899107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lazarini J.G., Massarioli A.P., Soares J.C., Nani B.D., Charo N., Oliveira D.S., Camargo L., Alvarez-Flores M.P., Batista I.D.F.C., Chudzinski-Tavassi A.M., Alencar S.M.D., Franchin M., Rosalen P.L. The phytoactive constituents of Eugenia selloi B.D. Jacks (pitangatuba): toxicity and elucidation of their anti-inflammatory mechanism(s) of action. Food Chem.: Molecular Sciences. 2022;4 doi: 10.1016/j.fochms.2022.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bai J., Zhang Y., Tang C., Hou Y., Ai X., Chen X., Zhang Y., Wang X., Meng X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110985. [DOI] [PubMed] [Google Scholar]
- 68.Mozaffari Godarzi S., Valizade Gorji A., Gholizadeh B., Mard S.A., Mansouri E. Antioxidant effect of p-coumaric acid on interleukin 1-β and tumor necrosis factor-α in rats with renal ischemic reperfusion. Nefrologia. 2020;40:311–319. doi: 10.1016/j.nefro.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Huang C., Liu S., Bai J., Fan X., Guo J., Jia Y., Zhang Z., Chen X., Jia Y., Zhang P., Wang B., Zhang X. Effects of quercetin on intracavernous pressure and expression of nitrogen synthase isoforms in arterial erectile dysfunction rat model. Int. J. Clin. Exp. Med. 2015;8:7599–7605. [PMC free article] [PubMed] [Google Scholar]
- 70.Xueni W., Junjie Z., Huimin Y., Mengyao S., Qiaoqi Z., Yu W., Yan Z., Lin M., Xiumei G. Kaempferol inhibits benign prostatic hyperplasia by resisting the action of androgen. Eur. J. Pharmacol. 2021;907 doi: 10.1016/j.ejphar.2021.174251. [DOI] [PubMed] [Google Scholar]
- 71.Kopalli S.R., Yoo S.K., Kim B., Kim S.K., Koppula S. vol. 27. 2022. (Apigenin Isolated from Carduus Crispus Protects against H(2)O(2)-Induced Oxidative Damage and Spermatogenic Expression Changes in GC-2spd Sperm Cells). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saima, Anjum I., Mobashar A., Jahan S., Najm S., Nafidi H.A., Bin Jardan Y.A., Bourhia M. Spasmolytic and Uroprotective Effects of Apigenin by Downregulation of TGF-β and iNOS Pathways and Upregulation of Antioxidant Mechanisms: In Vitro and In Silico Analysis. Pharmaceuticals (Basel) 2023;16(6):811. doi: 10.3390/ph16060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H. Effect of apigenin on the reproductive system in male mice. Health. 2010;2:435–440. [Google Scholar]
- 74.Rotimi D.E., Olaolu T.D., Adeyemi O.S. Pharmacological action of quercetin against testicular dysfunction: a mini review. Journal of Integrative Medicine. 2022;20:396–401. doi: 10.1016/j.joim.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Bharti S., Misro M.M., Rai U. Quercetin supplementation restores testicular function and augments germ cell survival in the estrogenized rats. Mol. Cell. Endocrinol. 2014;383:10–20. doi: 10.1016/j.mce.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 76.Adeoye O.O., Opeyemi A., Olawumi J., Adebanji A., Kehinde S.O., Emmanuel D.A., Olorunfemi T. Regulatory effects of quercetin on testicular histopathology induced by cyanide in Wistar rats. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tvrdá E., Kováč J., Ferenczyová K., Kaločayová B., Ďuračka M., Benko F., Almášiová V., Barteková M. Quercetin ameliorates testicular damage in zucker diabetic fatty rats through its antioxidant, anti-inflammatory and anti-apoptotic properties. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232416056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olawumi F.A.O., Opeyemi S.O. L-Arginine, Quercetin and Their Combination on Testicular Function and Sexual Behaviors in Rats. Journal of Infertility and Reproductive Biology. 2020:90–98. [Google Scholar]
- 99.Bancroft E.T.A.L. seventh ed. Churchill Livingstone Elsevier; 2013. Bancroft's Theory and Practice of Histological Techniques. [Google Scholar]
Further reading
- 51.Zhang X.R., Zhang Z.J., Jenkins T.A., Cheng W.R., Reynolds G.P. The dose-dependent effect of chronic administration of haloperidol, risperidone, and quetiapine on sexual behavior in the male rat. J. Sex. Med. 2011;8:3345–3353. doi: 10.1111/j.1743-6109.2010.01740.x. [DOI] [PubMed] [Google Scholar]
- 78.El-Sahhar K.F., Nassar D.M., Amer W.M., Qasem L.A. Morphological and anatomical studies of A.Saligna (labill.) wendl. The dominant plant species in AL-AHRASH PROTECTORATE-RAFAH-NORTH sinai-Egypt. Egyptian Journal of Agricultural Sciences. 2009;60(1):43–60. [Google Scholar]
- 79.hazrati S., Ebadi M.T., Mollaei S., Khurizadeh S. Evaluation of volatile and phenolic compounds, and antioxidant activity of different parts of Ferulago angulata (schlecht.) Boiss. Ind. Crop. Prod. 2019;140 doi: 10.1016/j.indcrop.2019.111589. [DOI] [Google Scholar]
- 80.Ilgin S., Burukoglu D., Baysal M., Hinis O., Atli O. Assessment of hepatotoxic effects of quetiapıne at repeated doses in rats. Anadolu University Journal of Science and Technology C - Life Sciences and Biotechnology. 2018;7(2):196–206. doi: 10.18036/aubtdc.349864. [DOI] [Google Scholar]
- 81.Klein-Schwartz W., Schwartz E.K., Anderson B.D. Evaluation of QET abuse and misuse reported to poison centers. J. Addiction Med. 2014;8(3):195–198. doi: 10.1097/ADM.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 82.Oruch R., Pryme I., Fasmer O.B., Lund A. EC PHARMACOLOGY AND TOXICOLOGY Review Article QET: An Objective Evaluation of Pharmacology, Clinical Uses and Intoxication. 2020;8:1–26. [Google Scholar]
- 83.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5(3):93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.WHO Drugs. 2019 https://www.who.int/teams/mental-health-and-substance-use/alcohol-drugs-and-addictive-behaviours/drugs-psychoactive [Google Scholar]
- 85.Behdarvand-Margha Z., Ahangarpour A. The Effects of Gallic Acid and Metformin on Male Reproductive Dysfunction in Diabetic Mice Induced by Methylglyoxal: an Experimental Study. 2021;vol. 19:715–724. doi: 10.18502/ijrm.v19i8.9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Correll C.U., Detraux J., De Lepeleire J., De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatr. 2015;14:119–136. doi: 10.1002/wps.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crespo I., García-Mediavilla M.V., Gutiérrez B., Sánchez-Campos S., Tuñón M.J., González-Gallego J. A comparison of the effects of kaempferol and quercetin on cytokine-induced proinflammatory status of cultured human endothelial cells. Br. J. Nutr. 2008;100:968–976. doi: 10.1017/S0007114508966083. [DOI] [PubMed] [Google Scholar]
- 88.De Berardis D., Rapini G., Olivieri L., Di Nicola D., Tomasetti C., Valchera A., Fornaro M., Di Fabio F., Perna G., Di Nicola M. Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine. Therapeutic advances in drug safety. 2018;9:237–256. doi: 10.1177/2042098618756261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jalili C., Korani M., Pazhouhi M., Ghanbari A., Zhaleh M., Davoudi S., Rashidi I. Protective effect of gallic acid on nicotine-induced testicular toxicity in mice. Res Pharm Sci. 2021;16:414–424. doi: 10.4103/1735-5362.319579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaya K., Ciftci O., Cetin A., Doğan H., Başak N. Hesperidin protects testicular and spermatological damages induced by cisplatin in rats. Andrologia. 2015;47:793–800. doi: 10.1111/and.12332. [DOI] [PubMed] [Google Scholar]
- 91.Liu T., Zhang L., Joo D., Sun S.C. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mehraban Z., Ghaffari Novin M., Golmohammadi M.G., Sagha M., Ziai S.A., Abdollahifar M.A., Nazarian H. Protective effect of gallic acid on testicular tissue, sperm parameters, and DNA fragmentation against toxicity induced by cyclophosphamide in adult NMRI mice. Urol. J. 2020;17:78–85. doi: 10.22037/uj.v0i0.4858. [DOI] [PubMed] [Google Scholar]
- 94.Ortega-Ruiz M., Soria-Chacartegui P., Villapalos-García G., Abad-Santos F., Zubiaur P. The pharmacogenetics of treatment with QET. Future Pharmacology. 2022;2 [Google Scholar]
- 95.Owumi S.E., Adedara I.A., Akomolafe A.P., Farombi E.O., Oyelere A.K. Gallic acid enhances reproductive function by modulating oxido-inflammatory and apoptosis mediators in rats exposed to aflatoxin-B1. Exp Biol Med (Maywood) 2020;245:1016–1028. doi: 10.1177/1535370220936206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oyeleye S.I., Adefegha S.A., Dada F.A., Okeke B.M. Effect of P-Coumaric Acid on the Erectogenic Enzyme Activities and Non-protein Thiol Level in the Penile Tissue of Normal and Doxorubicin-Induced Oxidative Stress Male Rat. 2019;vol. 51 doi: 10.1111/and.13281. [DOI] [PubMed] [Google Scholar]
- 97.Roychoudhury S., Agarwal A., Virk G., Cho C.-L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online. 2017;34:487–498. doi: 10.1016/j.rbmo.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 98.Wang F.-F., Wang Q., Chen Y., Lin Q., Gao H.-B., Zhang P. Chronic stress induces ageing-associated degeneration in rat Leydig cells. Asian J. Androl. 2012;14:643. doi: 10.1038/aja.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elansary H.O., Szopa A., Kubica P., Ekiert H., A. AL-Mana F., AL-Yafrsi M.A. Antioxidant and biological activities of Acacia saligna and lawsonia inermis natural populations. Plants. 2020;9:908. doi: 10.3390/plants9070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grolli R.E., Bertollo A.G., Behenck J.P., De Araujo Borba L., Plissari M.E., Soares S.J.B., Manica A., Da Silva Joaquim L., Petronilho F., Quevedo J., Bagatini M.D., Réus G.Z., Ignácio Z.M. Quetiapine effect on depressive-like behaviors, oxidative balance, and inflammation in serum of rats submitted to chronic stress. N. Schmied. Arch. Pharmacol. 2023 doi: 10.1007/s00210-023-02406-8. [DOI] [PubMed] [Google Scholar]
- 102.Heilbronn C., Lloyd B., Mcelwee P., Eade A., Lubman D.I. Trends in quetiapine use and non‐fatal quetiapine‐related ambulance attendances. Drug Alcohol Rev. 2013;32:405–411. doi: 10.1111/dar.12028. [DOI] [PubMed] [Google Scholar]
- 103.Korkut Celikates B., Kilic V., Atli-Eklioglu O. vol. 45. 2022. pp. 2379–2387. (Effects of Quetiapine Administration on Sperm Quality and Testicular Histology). [DOI] [PubMed] [Google Scholar]
- 104.Mattson M.E., Albright V.A., Yoon J., Council C.L. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: results from the Drug Abuse Warning Network (DAWN) Subst. Abuse Res. Treat. 2015;9 doi: 10.4137/SART.S22233. SART. S22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Midgley S., Turnbull J. Domestication and use of Australian acacias: case studies of five important species. Aust. Syst. Bot. 2003;16:89–102. [Google Scholar]
- 106.Poyurovsky M., Braverman L., Weizman A. Beneficial effect of quetiapine monotherapy in patients with bipolar depression and comorbid obsessive-compulsive disorder. Int. Clin. Psychopharmacol. 2020;36:50–53. doi: 10.1097/YIC.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 107.Vilibić M., Peitl V., Živković M., Vlatković S., Ljubičić Bistrović I., Ljubičić R., Matošić A., Karlović D. Quetiapine add-on therapy may improve persistent sleep disturbances in patients with PTSD on stabile combined SSRI and benzodiazepine combination: a one-group pretest-posttest study. Psychiatr. Danub. 2022;34:245–252. doi: 10.24869/psyd.2022.245. [DOI] [PubMed] [Google Scholar]
- 108.ZHANG X.R., ZHANG, CHENG W.R., REYNOLDS G.P. The dose-dependent effect of chronic administration of haloperidol, risperidone, and QET on sexual behavior in the male rat. J Sex Med. 2011;8:3345–3353. doi: 10.1111/j.1743-6109.2010.01740.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.