Abstract
Human herpesvirus 6 (HHV-6), which belongs to the betaherpesvirus subfamily and infects mainly T cells in vitro, causes acute and latent infections. Two variants of HHV-6 have been distinguished on the basis of differences in several properties. We have determined the complete DNA sequence of HHV-6 variant B (HHV-6B) strain HST, the causative agent of exanthem subitum, and compared the sequence with that of variant A strain U1102. A total of 115 potential open reading frames (ORFs) were identified within the 161,573-bp contiguous sequence of the entire HHV-6 genome, including some genes with remarkable differences in amino acid identity. All genes with <70% identity between the two variants were found to contain deleted regions when ORFs that could not be expressed were excluded from the comparison. Except in the case of U47, these differences were found in immediate-early/regulatory genes, DR2, DR7, U86/90, U89/90, and U95, which may represent characteristic differences of variants A and B. Also, we have successfully typed 14 different strains belonging to variant A or B by PCR using variant-specific primers; the results suggest that the remarkable differences observed were conserved evolutionarily as variant-specific divergence.
Human herpesvirus 6 (HHV-6) was first isolated in 1986 from the peripheral blood of patients with lymphoproliferative disorders (53). By comparison with other human herpesviruses, molecular and immunological analyses revealed its distinct nature (22). The virus replicates predominantly in CD4+ lymphocytes (33, 60) and may establish latent infection in cells of the monocyte/macrophage lineage (23). Infection with this virus results in exanthem subitum (ES), a common illness of infancy (74), but has not yet been clearly linked to any disease in adults. Two variants of HHV-6 have been identified on the basis of differences in epidemiology, in vitro growth properties, reactivity with monoclonal antibodies, restriction endonuclease mapping, and nucleotide sequence (1, 2, 8, 15, 55, 72, 73), leading to adoption of the nomenclature HHV-6A (variant A) and HHV-6B (variant B). HHV-6A has not yet been clearly linked to any disease, and HHV-6B is the causative agent of ES (3). Gompels and coworkers (16) have determined the complete DNA sequence of HHV-6A strain U1102. In the present report, to examine which gene(s) could be related to pathogenicity and other viral properties, we present the complete DNA sequence of HHV-6 strain HST, which belongs to variant B, and compare the sequence with that of HHV-6A. We also report that some of the HHV-6 genes show remarkable differences in deduced amino acid sequence identity compared with variant A. Such differences could represent characteristic, or possibly pathogenic, differences between the two variants.
MATERIALS AND METHODS
Cells and viruses.
Umbilical cord blood mononuclear cells (CBMCs) were separated on a Ficoll-Conray gradient, cultured in RPMI 1640 medium containing 10% fetal calf serum, and stimulated with phytohemagglutinin (5 μg/ml) for 2 or 3 days. HHV-6 strain HST, which was isolated from a patient with ES and belongs to the variant B group, was propagated in fresh human peripheral mononuclear cells (74). To prepare virus stocks, virus was propagated in stimulated CBMCs. When more than 80% of the cells showed cytopathic effects, the culture of infected cells was frozen and thawed twice; after centrifugation at 1,500 × g for 10 min, the supernatant fraction was stored at −80°C as a cell-free virus stock. To infect cells with virus, stimulated CBMCs (107 cells) were washed twice with phosphate-buffered saline, suspended in 1 ml of virus solution with 107 50% tissue culture infective doses per ml, and spun at 1,500 × g for 40 min at 37°C for adsorption. To prepare the viral DNA, the infected cells were cultured for 2 or 3 days in RPMI 1640 medium supplemented with 10% fetal calf serum and harvested when approximately 50% of the cells showed cytopathic effects. For analysis of independently isolated viruses by PCR, CBMCs infected with the viruses were used. The following viruses were kindly supplied by other researchers: strain Z29 by C. Lopez (32), strains GS and DA by D. V. Ablashi (1), strain U1102 by R. Honess (11), and strain St. W. by G. Enders (12). Other HHV-6 strains (MNM1, MNM2, TMK1, TMK2, NKT2, OKC1, OKC3, and MCZ2) were isolated in our laboratory from patients with ES. All strains were confirmed as HHV-6 by using specific monoclonal antibodies to HHV-6 (data not shown). To test the specificities of the primers used for conducting virus strain typing, herpes simplex virus type 1 (HSV-1; strain F), HSV-2 (strain G), varicella-zoster virus (strain Oka), human cytomegalovirus (HCMV; strain AD169), Epstein-Barr virus (strain B95-8), and HHV-7 (strain KHR) were used for PCR.
Preparation and purification of HHV-6 DNA.
To determine the DNA sequence of the entire HHV-6 HST genome, viral genomic DNA was purified as described elsewhere (35, 43). To amplify HHV-6 genome DNA by PCR, DNA from HHV-6-infected CBMCs (106 cells) 2 days after infection was extracted in 0.5 ml of lysis buffer (0.45% Nonidet P-40, 0.45% Tween, 10 mM Tris-HCl [pH 8.3], 50 mM KCl, 3 mM MgCl2, 10 μg of protease K per ml) at 65°C overnight and at 98°C for 10 min to denature the proteinase K.
PCR.
For assembling the various DNA sequences into the complete genome DNA sequence, the junction regions of nonoverlapping sequences, except the origin of DNA binding (Ori) region, the 3′ end of the unique region, and the concatemer junction region, were amplified by PCR using genomic viral DNA prepared as described above with corresponding oligonucleotide primers, which were synthesized based on the previously determined HHV-6 sequence at the fragment termini. PCR was carried out with approximately 10 ng of the viral DNA template as follows: 25 cycles of denaturation (95°C for 1 min), annealing (55°C for 1 min), and extension (72°C for 2 min), using a PCR Thermal Cycler TP480 (Takara Shuzo, Kyoto, Japan). For the Ori region and the right-hand end of the unique region, amplified DNA fragments were obtained from 10 ng of purified strain HST DNA by shuttle PCR as described below, using LA Taq (Takara Shuzo), an appended buffer, 200 μM each deoxynucleoside triphosphate, and two pairs of PCR primers, 06-3end (5′-AAGACACCATACTCGGAAGAATCATGTTACGACGTT)–22-5end (5′-TTGGCCTGAGACCTTACAACGGAGACGCCCGTGATC) and 07-5end (5′-GAGATAGAGTCGGCCTTGGAACACGTCGTGGCAGAAC)–45-3end (5′-GATAAAAAAGTAATTCGTTCATATGAGTAGTCGGAT), respectively. In the case of the concatemer junction region, the DNA fragment was obtained from extracted viral DNA by shuttle PCR as described below, using primers 5Ter-JuncC.LA (5′-CAGGGCGTGGCTGACACGCGCCGGCAGCGGCAT) and 3Ter-JuncF.LA (5′-ACGACGCCAAGGGAAGCCTCTGGCGCAATCTAT). The PCR product was then cloned into vector pCR2.1 (Invitrogen, San Diego, Calif.). Shuttle PCR conditions were as follows: incubation at 98°C for 3 min for hot start, 30 cycles of 98°C for 30 s, and 68°C for 10 min, with a final extension at 72°C for 10 min.
DNA sequencing and sequence analysis.
Purified viral genomic DNA was digested with restriction enzymes PstI, BamHI, HindIII, or KpnI (Takara Shuzo). The resultant fragments were cloned into the corresponding restriction enzyme sites of pUC19. To make deletion libraries, pUC19 clones carrying PstI-DNA fragments (designated pSTY clones) and the other restriction fragments (Fig. 1) were subjected to unidirectional progressive deletions, except for pSTY57, which contained an insert of approximately 200 bp. This procedure was carried out with a deletion kit for kilo-sequencing (Takara Shuzo), and the DNAs were subsequently transformed into Escherichia coli to obtain the corresponding deletion mutants. These inserts, as well as the normal PCR products described above, were sequenced as described by Isegawa et al. (20). Their corresponding sequences were confirmed by using the opposite unidirectional progressive deletions of the pSTY clones, the cassette-ligation-mediated PCR method, or direct sequencing of PCR products amplified from DNA (20). The resultant shuttle PCR products mentioned above were directly sequenced by cassette-ligation-mediated PCR (20) using a Li-Cor (Lincoln, Neb.) 4000L DNA sequencer. The nucleotide sequence of the entire HHV-6 genome thus obtained was analyzed to identify potential open reading frames (ORFs), using the software packages MacDNASIS version 3.7 (Hitachi Soft Engineering Co., Ltd., Yokohama, Japan) and ALIGN (Genome Information Research Center, Osaka University). Sequence comparisons were carried out using the sequences of HHV-6A (strain U1102) (16), HHV-6B (strain Z29) (9, 26, 27, 48), and HHV-7 (strain JI) (44).
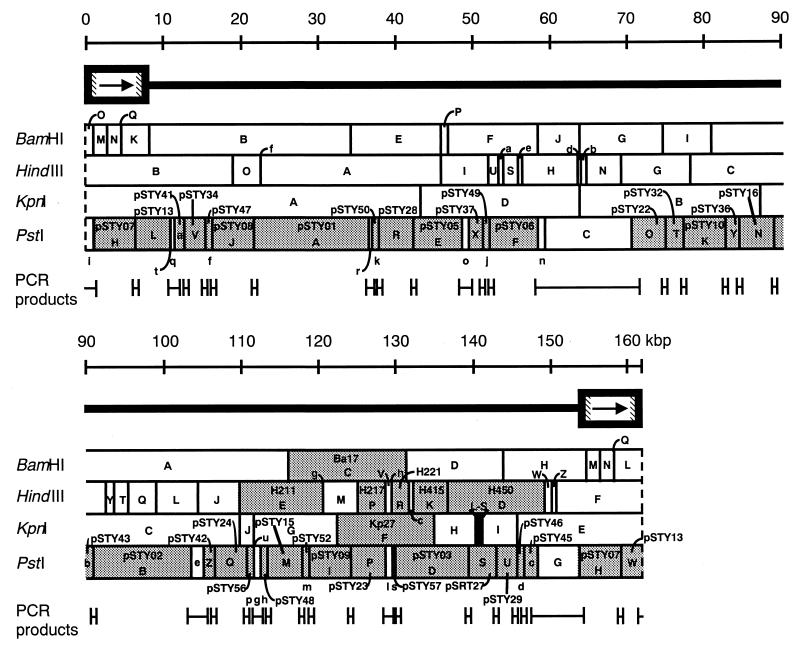
FIG. 1.
Restriction fragments used for the sequencing of HHV-6 strain HST. The HHV-6 genome is represented diagrammatically, together with the positions of BamHI, HindIII, KpnI, and PstI sites. Various plasmid-cloned restriction fragments are prefixed with Ba, H, Kp, or pSTY (indicating their terminal restriction sites). Sequenced restriction fragments are indicated by shading.
Nucleotide sequence accession number.
The complete sequence of HHV-6 strain HST has been deposited in the GenBank, EMBL, and DDBJ Data Libraries under accession no. AB021506.
RESULTS
Assembling the contiguous HHV-6B sequence.
The HHV-6 genome was sequenced by using the PstI, BamHI, HindIII, and KpnI deletion libraries described above. The fragments used for the project are shown in Fig. 1. The resultant PstI-DNA fragments of 15.3, 13.1, 9.7, 6.6, 6.5, 5.5, 5.3, 5.3, 5.2, 4.8, 4.6, 4.5, 4.4, 4.4, 4.1, 3.8, 4.0, 2.5, 2.7, 2.1, 1.7, 1.7, 1.5, 1.5, 1.5, 1.4, 1.2, 1.2, 1.1, 1.0, 1.0, 0.8, 0.7, and 0.2 kb were cloned into pUC19 and designated pSTY01, -02, -03, -05, -06, -07, -08, -09, -10, -13, -15, -16, -22, -23, -24, -27, -28, -29, -32, -34, -36, -37, -41, -42, -43, -45, -46, -47, -48, -49, -50, -52, -56, and -57, respectively. These pSTY clones covered about 85% of the HHV-6 genome. Junctions between nonoverlapping restriction fragments were determined by directly sequencing PCR fragments spanning the junction regions (see Materials and Methods). The sequences of the terminal direct repeat (DR) elements were derived by compiling partial sequences of DRL and DRR to generate the complete DR sequences. The positions of the left and right genome termini were determined by sequencing PCR fragments that had been produced with 5′- and 3′-terminal concatemer junction primers (see Materials and Methods). As shown in Fig. 2, the HHV-6 genome was composed of the DRs at the left and right ends of the genome (DRL and DRR) and the unique region (UR; containing the repeat regions [R1, R2, and R3] and Ori), for a total of 161,573 bp, including 8,231 bp in the DRs (DRL, nucleotide positions 1 to 8231; DRR, nucleotide positions 153,333 to 161573) and 145,101 bp in the UR (nucleotide positions 8232 to 153332). The genome length of the HST strain was 2,244 bp larger than that of the U1102 strain (144 and 1,966 bp larger at DR and UR, respectively). The overall GC content of the HST strain was 43%, the same as that of the U1102 strain. When the nucleotide sequence was compared with that of the U1102 strain, marked differences were observed only at specific regions of the DR and at the junction between the UR and DR. Higher similarity was observed in the UR. The nucleotide sequence variation of these regions is attributable differences between strains rather than differences between variants (43).
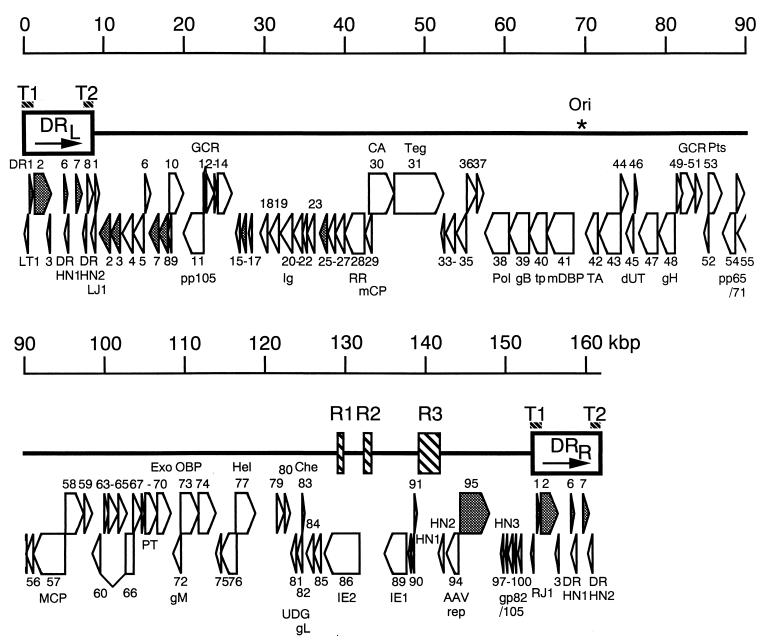
FIG. 2.
Predicted ORF organization of HHV-6 strain HST. Repeat regions (DRL, DRR, R1, R2, and R3) are boxed; telomeric repeat regions (T1 and T2) are denoted by striped bars; UR is indicated by a solid line. Protein coding regions are indicated as open arrows and are numbered DR1, DR2, DR3, DR6, DR7, DR8, DRHN1, and DRHN2 within the direct repeats and HN1, HN2, HN3, and U1 to U100 (excluding U78, U88, U92, U93, and U96) within the UR. The Ori is indicated by an asterisk. These regions are further described in Table 1 and in the text. The US22 gene family is shaded. Abbreviations not given in text: GCR, G-protein-coupled receptor; Ig, Ig immunoglobulin superfamily; RR, large subunit of ribonucleotide reductase; mCP, minor capsid protein; CA, capsid assembly protein; Teg, tegument protein; Pol, DNA polymerase; tp, transport protein; mDBP, major single-stranded DNA binding protein; TA, conserved herpesvirus transactivator; dUT, dUTPase; Pts, protease/assembly protein; pp65/71, phosphoprotein 65/71K; MCP, major capsid protein; PT, phosphotransferase; Exo, exonuclease; OBP, origin binding protein; Hel, helicase; UDG, uracil-DNA glycosylase; Che, chemokine; AAV rep1, adeno-associated virus replication protein homolog.
Identification of HHV-6B ORFs.
The contiguous 161,573-bp sequence of the entire HHV-6 genome was analyzed to identify potential ORFs, using DNASIS version 3.7 (Hitachi Soft Engineering). Potential genes in the HHV-6 strain HST genome were selected by the following criteria: (i) ORFs larger than 300 nucleotides with initiating methionine codons (ATG) were generally considered to be genes encoding proteins; and (ii) ORFs smaller than 300 nucleotides but larger than 80 nucleotides were considered to be genes if their deduced amino acid sequences showed significant homology to those of U1102 and other herpesviruses or cellular proteins. The ORFs and homologous regions thus identified are illustrated in Fig. 2 and listed in Table 1. Within the DR, there are several ORFs (LT1, DR1, DR2, DR3, DR6, DR7, DR8, DRHN1, and DRHN2), and numerous ORFs are present within the UR. In general, the degrees of identity between corresponding ORFs were very high between variants A and B (Table 1). However, variant-specific ORFs were observed in the genome of each strain: HN1, HN2, HN3, DRHN1, and DRHN2 in the HST strain and U78, U88, U92, U93, U96, DR4, and DR5 in the U1102 strain. HN1 and HN3 turned out to be exons of the U89-IE1 (immediate-early 1) and spliced glycoprotein gp82/105 genes, respectively, when their cDNAs were analyzed. The functions of these variant-specific ORFs are not clear. It is possible that some ORFs may not encode functional proteins at all. However, it is conceivable that the others are associated with biological and pathogenic differences between variants A and B.
TABLE 1.
ORF identity between HST and U1102 strains of HHV-6
| ORFa
|
Orientation | Position
|
N-terminal Met?b | Length (aa)c | Identity (%)d | Z29 ORFe | Identity (%)f | HHV-7 homologe | Comments | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HST | U1102 | Start | Stop | Poly(A) signalg | ||||||||
| LT1 | LT1 | − | 364 | 17 | Y | 115/112 | 52.5 | |||||
| DR1 | DR1 | + | 576 | 842 | 3912 | Y | 88/97 | 86.4 | DR1 | US22 gene family | ||
| DR2 | DR2 | + | 1027 | 2970 | 3912 | Y | 647/620 | 68.2 | DR2 | US22 gene family | ||
| DR3 | DR3 | − | 3320 | 2718 | Y | 200/192 | 82.5 | |||||
| DR4 | NAh/100 | |||||||||||
| DR5 | NA/145 | |||||||||||
| DR6 | DR6 | + | 5025 | 5336 | 5952 | Y | 103/103 | 84.5 | DR6 | US22 gene family | ||
| DRHN1 | − | 5532 | 5023 | 4745 | Y | 169/NA | ||||||
| DR7 | DR7 | + | 6512 | 7150 | 7468 | Y | 212/363 | 42.2 | DR7 | US22 gene family, transformation, transactivator | ||
| DRHN2 | − | 7706 | 7236 | 4745 | Y | 156/NA | ||||||
| DR8 | DR8 | + | 7928 | 8662 | Y | 244/110 | 13.6 | |||||
| LJ1 | LJ1 | − | 8807 | 8292 | Y | 172/321 | 16.6 | |||||
| U1 | U1 | + | 8929 | 9384 | Y | 151/123 | 25.6 | |||||
| U2 | U2 | − | 10768 | 9467 | 9360 | Y | 433/366 | 92.0 | U2 | US22 gene family | ||
| U3 | U3 | − | 12051 | 10891 | 9360 | Y | 386/373 | 90.1 | U3 | US22 gene family | ||
| U4 | U4 | − | 13883 | 12276 | 9360 | Y | 535/535 | 98.1 | U4 | |||
| U5 | U5 | − | 15333 | 14002 | 13919 | Y | 443/444 | 94.6 | U5/7 | |||
| U6 | U6 | + | 15395 | 15652 | 16088 | N | 85/82 | 95.1 | ||||
| U7 | U7 | − | 16802 | 15678 | 15447* | Y | 374/342 | 97.3 | US22 gene family | |||
| U8 | U8 | − | 18041 | 16806 | 15447 | Y | 411/356 | 83.0 | U8 | US22 gene family | ||
| U9 | U9 | − | 18336 | 18022 | 17999 | Y | 104/104 | 94.2 | ||||
| U10 | U10 | + | 18386 | 19897 | 20435* | Y | 503/436 | 95.6 | U10 | |||
| U11 | U11 | − | 22377 | 19801 | 19765 | Y | 858/870 | 80.1 | p100 | 100 | U11 | pp100, major antigenic structural protein |
| U12EX | U12EX | + | 22479 | 22553 | Y | 353/347 | 88.4 | U12EX | U12 exon 1, spliced donor 22511 | |||
| U12 | U12 | + | 22700 | 23617 | 23634 | Y | 305/318 | 90.2 | U12 | CC-chemokine receptor, exon 2, acceptor 22589 | ||
| U13 | U13 | + | 23699 | 24022 | 24775 | Y | 107/106 | 92.5 | U13 | |||
| U14 | U14 | + | 24136 | 25953 | 26012 | Y | 610/609 | 86.0 | U14 | |||
| U15 | U15 | − | 26891 | 26559 | 26300* | Y | 110/110 | 88.2 | U15 | |||
| U16 | U16 | − | 27603 | 27172 | 27105 | Y | 143/143 | 94.4 | U16 | IE-B, transactivator, US22 gene family | ||
| U17 | U17 | − | 28263 | 28003 | 27975 | Y | 86/133 | 95.3 | U17 | IE-B | ||
| U18 | U18 | − | 30327 | 29443 | 29447i | Y | 294/293 | 88.7 | U18 | IE-B, homology to HCMV IE glycoprotein | ||
| U19 | U19 | − | 31761 | 30592 | 29447 | Y | 389/389 | 91.5 | U19 | IE-B | ||
| U20 | U20 | − | 33288 | 31984 | 31940 | Y | 434/422 | 91.0 | U20 | Glycoprotein, Ig chain C domain | ||
| U21 | U21 | − | 34793 | 33291 | 31940 | Y | 500/433 | 89.8 | U21 | Glycoprotein | ||
| U22 | U22 | − | 35298 | 34690 | 34656* | Y | 202/202 | 95.5 | Glycoprotein | |||
| U23 | U23 | − | 36225 | 35326 | 35010 | Y | 299/236 | 86.9 | U23 | Glycoprotein | ||
| U24 | U24 | − | 36616 | 36350 | 36253* | Y | 88/87 | 81.8 | U24 | |||
| U25 | U25 | − | 37775 | 36825 | 36253* | Y | 316/316 | 98.1 | U25 | US22 gene family | ||
| U26 | U26 | − | 38770 | 37883 | 37847 | Y | 295/295 | 92.9 | U26 | |||
| U27 | U27 | − | 39933 | 38758 | 37847 | Y | 391/393 | 95.4 | U27 | pp41, DNA polymerase processivity transactivator | ||
| U28 | U28 | − | 42389 | 39975 | 39968 | Y | 804/804 | 96.0 | U28 | Large subunit of ribonucleotide reductase | ||
| U29 | U29 | − | 43311 | 42412 | 42365 | Y | 299/299 | 96.0 | U29 | Minor capsid protein | ||
| U30 | U30 | + | 42839 | 46087 | 49520 | Y | 1082/1082 | 92.4 | U30 | Capsid assembly, myosin | ||
| U31 | U31 | + | 46105 | 52338 | 52395 | Y | 2077/2077 | 93.0 | U31 | Large tegument protein | ||
| U32 | U32 | − | 52681 | 52412 | 52380 | Y | 89/88 | 95.5 | U32 | |||
| U33 | U33 | − | 54095 | 52683 | 52380 | Y | 470/470 | 98.3 | U33 | Capsid protein | ||
| U34 | U34 | − | 54876 | 54046 | 53960 | Y | 276/276 | 93.8 | U34 | Possible virion protein | ||
| U35 | U35 | − | 55210 | 54893 | 54255 | Y | 106/106 | 96.2 | U35 | |||
| U36 | U36 | + | 55212 | 56660 | 57454* | Y | 481/484 | 95.0 | U36 | Probable virion protein | ||
| U37 | U37 | + | 56664 | 57458 | 57454*i | Y | 264/264 | 97.0 | U37 | |||
| U38 | U38 | − | 60542 | 57504 | 57469 | Y | 1012/1012 | 97.5 | U38 | DNA polymerase | ||
| U39 | U39 | − | 63034 | 60541 | 60423 | Y | 830/830 | 96.1 | gB | 98.2 | U39 | gB |
| U40 | U40 | − | 65168 | 62988 | 62578 | Y | 726/726 | 98.3 | KA1L | 100 | U40 | Transport protein |
| U41 | U41 | − | 68574 | 65176 | 64037* | Y | 1132/1132 | 98.8 | KA2L | 100 | U41 | Major DNA binding protein |
| U42 | U42 | − | 71487 | 69937 | 69804 | Y | 516/514 | 95.2 | KA3L | 100 | U42 | Conserved herpesvirus transactivator |
| U43 | U43 | − | 74294 | 71712 | 71615* | Y | 860/860 | 97.7 | KA4L | 100 | U43 | Helicase/primase complex, HSV primase |
| U44 | U44 | + | 74335 | 75030 | 75062* | Y | 231/213 | 95.5 | KA5R | 100 | U44 | |
| U45 | U45 | − | 76107 | 74977 | 74714* | Y | 376/376 | 94.4 | KA6L | 100 | U45 | Putative dUTPase |
| U46 | U46 | + | 76180 | 76434 | 76479 | Y | 84/84 | 94.0 | KA7R | 100 | U46 | |
| U47 | U47 | − | 78833 | 76617 | 76578 | Y | 738/651 | 69.8 | KA8L | 99.9 | U47 | |
| U48 | U48 | − | 81183 | 79099 | 79099i | Y | 694/694 | 94.4 | KA9L | 99.9 | U48 | gH |
| U49 | U49 | + | 81342 | 82100 | 84529 | Y | 252/252 | 97.2 | KA10R | 100 | U49 | Fusion protein |
| U50 | U50 | + | 81877 | 83544 | 84529 | Y | 555/555 | 97.3 | KA11R | 100 | U50 | Virion protein |
| U51 | U51 | + | 83642 | 84547 | 84579 | Y | 301/301 | 94.0 | KA12R | 100 | U51 | G-protein-coupled receptor homolog |
| U52 | U52 | − | 85343 | 84744 | 84570 | Y | 258/258 | 96.9 | U52 | |||
| U53 | U53 | + | 85350 | 86936 | 87101 | Y | 528/528 | 96.6 | U53 | Protease, in-frame assembly protein | ||
| U54 | U54 | − | 88550 | 87171 | 87142 | Y | 459/458 | 79.5 | U54 | Tegument transactivator, pp65/72K | ||
| U55 | U55 | − | 90106 | 88628 | 88485* | Y | 492/432 | 93.3 | U55 | |||
| U56 | U56 | − | 90997 | 90107 | 90111*i | Y | 296/296 | 97.6 | U56 | Capsid protein | ||
| U57 | U57 | − | 95036 | 90999 | 90236* | Y | 1345/1345 | 97.6 | U57 | Major capsid protein | ||
| U58 | U58 | + | 95048 | 97366 | 98648* | Y | 772/772 | 97.3 | U58 | |||
| U59 | U59 | + | 97375 | 98415 | 98648* | Y | 350/350 | 94.6 | U59 | |||
| U60 | U60 | − | 99380 | 98412 | 97688* | Y | 322/322 | 100 | U60 | Late spliced gene (U60/66) for DNA packaging | ||
| U61 | U61 | − | 99867 | 99355 | 99089 | N | 170/115 | 94.8 | ||||
| U62 | U62 | + | 99551 | 99814 | 100161* | Y | 87/85 | 92.0 | U62 | |||
| U63 | U63 | + | 99756 | 100412 | 100563* | Y | 218/216 | 98.6 | U63 | |||
| U64 | U64 | + | 100390 | 101718 | 102152 | Y | 442/442 | 93.2 | U64 | |||
| U65 | U65 | + | 101675 | 102682 | 102721 | Y | 335/335 | 85.1 | U65 | |||
| U66 | U66 | − | 103619 | 102702 | 101283* | Y | 305/305 | 97.4 | U66 | Late spliced gene (U60/66) for DNA packaging | ||
| U67 | U67 | + | 103591 | 104652 | 104787* | Y | 353/353 | 96.6 | U67 | |||
| U68 | U68 | + | 104652 | 104996 | 106850 | Y | 114/114 | 93.9 | U68 | |||
| U69 | U69 | + | 104999 | 106690 | 106850 | Y | 563/562 | 94.1 | U69 | Ganciclovir kinase, conserved phosphotransferase | ||
| U70 | U70 | + | 106698 | 108164 | 108399 | Y | 488/488 | 96.3 | CH3R | 99.8 | U70 | Alkaline exonuclease |
| U71 | U71 | + | 108101 | 108346 | 108399 | Y | 81/77 | 85.2 | CH4R | 100 | U71 | |
| U72 | U72 | − | 109462 | 108428 | 108383 | Y | 344/344 | 97.1 | CH5L | 100 | U72 | Integral membrane protein, gM |
| U73 | U73 | + | 109475 | 111817 | 113835 | Y | 780/780 | 97.6 | CH6R | 99.9 | U73 | Origin binding protein |
| U74 | U74 | + | 111786 | 113774 | 113835 | Y | 662/662 | 96.5 | CB1R | 99.7 | U74 | Helicase/primase complex |
| U75 | U75 | − | 113756 | 113379 | 113163 | Y | 249/249 | 96.0 | CB2L | 100 | U75 | |
| U76 | U76 | − | 116455 | 114467 | 113720 | Y | 662/662 | 98.9 | CB3L | 99.7 | U76 | |
| U77 | U77 | + | 116250 | 118724 | 119241* | Y | 824/824 | 98.9 | CB4R | 99.8 | U77 | Helicase/primase complex, helicase |
| U78 | NA/109 | |||||||||||
| U79 | U79 | + | 121322 | 122359 | 123038 | Y | 345/344 | 77.4 | CB7R | 99.7 | U79 | HCMV replication, spliced |
| U80 | U80 | + | 122640 | 122942 | 123038 | Y | 203/198 | 81.0 | CB8R | 100 | U80 | HCMV replication, spliced |
| U81 | U81 | − | 123753 | 122986 | 122950 | Y | 255/255 | 94.1 | CB9L | 98.8 | U81 | Uracil-DNA glycosylase |
| U82 | U82 | − | 124581 | 123829 | 122950 | Y | 250/250 | 93.6 | CB10L | 99.6 | U82 | gL, gH accessory protein |
| U83 | U83 | + | 124657 | 124998 | 125133 | Y | 113/97 | 87.5 | CB11R | 100 | U83 | Chemokine |
| U84 | U84 | − | 126132 | 125104 | 125100 | Y | 342/342 | 90.9 | U84 | Spliced in HCMV | ||
| U85 | U85 | − | 127038 | 126160 | 126117 | Y | 292/290 | 90.8 | U85 | OX-2 homology, glycoprotein | ||
| U86 | U86 | − | 131717 | 127176 | 127157 | Y | 1513/1351 | 64.3 | U86 | IE-A, HCMV IE2 homolog, acceptor 131767 | ||
| U88 | NA/413 | |||||||||||
| U89 | U89 | − | 137684 | 134808 | 134579 | Y | 958/839 | 62.2 | IE1 | 92.3 | U89 | IE-A, HCMV IE1 position, transactivator, acceptor 137737 |
| U90 | U90 | − | 138085 | 137810 | 134579 | N | 89/94 | 61.6 | U90 | IE-A, spliced U86 and U89, acceptor/donor 138059/137848 | ||
| HN1 | − | 138241 | 138143 | 134579 | Y | 32/NA | IE-A, spliced U90, donor 138147 | |||||
| U91 | U91 | + | 138630 | 138974 | 139230* | N | 114/114 | 72.4 | U91 | |||
| U92 | NA/147 | |||||||||||
| U93 | NA/197 | |||||||||||
| HN2 | − | 142355 | 141543 | 141312* | Y | 270/NA | ||||||
| U94 | U94 | − | 144155 | 142683 | 142631 | Y | 490/490 | 97.6 | Parvovirus replication, transactivation | |||
| U95 | U95 | + | 144230 | 147868 | 147919* | Y | 1197/1121 | 64.0 | U95 | MCMV IE2 homolog, US22 gene family | ||
| U96 | NA/99 | |||||||||||
| U97 | U97 | − | 149651 | 149360 | 148258* | Y | 99/89 | 79.8 | Spliced glycoprotein gp82/105 | |||
| HN3 | − | 149913 | 149746 | 148258* | N | 55/NA | Spliced glycoprotein gp82/105 | |||||
| U98 | U98 | − | 150870 | 150376 | 148258* | Y | 164/216 | 78.6 | U98 | gp82/105 | ||
| U99 | U99 | − | 151396 | 151115 | 148258* | Y | 93/93 | 78.9 | U99 | gp82/105 | ||
| U100 | U100 | − | 151918 | 151529 | 148258* | Y | 129/189 | 72.1 | U100 | gp82/105 | ||
| RJ1 | RJ1 | − | 153407 | 153060 | Y | 116/112 | 57.5 | |||||
| DR1′ | DR1′ | + | 153618 | 153884 | 156954 | Y | 88/97 | 86.4 | DR1′ | US22 gene family | ||
| DR2′ | DR2′ | + | 154069 | 156012 | 156954 | Y | 647/620 | 74.8 | DR2′ | US22 gene family | ||
| DR3′ | DR3′ | − | 156362 | 155760 | Y | 200/192 | 82.5 | |||||
| DR4′ | NA/100 | |||||||||||
| DR5′ | NA/145 | |||||||||||
| DR6′ | DR6′ | + | 158067 | 158378 | 158994 | Y | 103/103 | 84.5 | DR6′ | US22 gene family | ||
| DRHN1′ | − | 158574 | 158065 | 157787 | Y | 169/NA | ||||||
| DR7′ | DR7′ | + | 159554 | 160192 | 160510 | Y | 212/363 | 82.4 | DR7′ | US22 gene family, transformation, transactivator | ||
| DRHN2′ | − | 160748 | 160278 | 157787 | Y | 156/NA | ||||||
| DR8′ | NA/110 | |||||||||||
HST ORFs are named after their U1102 homologs (16); ORFs unique to HST are prefixed with “HN” or “DRHN” and numbered 1 and 2.
Y, yes; N, no.
Of ORFs translation product (HST/U1102).
Values for percent identity between HST and U1102 homologs are based on ALIGN alignments, with gap and length weights set at 3.0 and 0.1, respectively.
Homologous genes were identified by database searches. Listings of homologous genes were based on these analyses and on data from comparisons of HHV-6 and HHV-7 strain JI (9, 26, 27, 44, 48).
The values for percent identity between HST and Z29 homologs are based on ALIGN alignments, with gap and length weights set at 3.0 and 0.1, respectively.
First downstream polyadenylation signals (AATAAA or ATTAAA*).
NA, not applicable.
Polyadenylation signals overlapping ORF C-terminal sequences.
Comparisons of ORFs between HHV-6 variants.
Sequence alignments of ORFs in the UR between the HST and U1102 strains indicated that the amino acid identity between these viruses was about 94% on average, except for the variant-specific ORFs. Putative functions of the various HHV-6 genes implicated by their corresponding homologs are listed in Table 1, which shows that 67 of the 103 ORFs compared exhibited >90% identity between these strains. As shown in Table 1, ORFs with 80 to 90% identity were DR1, DR3, DR6, U8, U11, U12, U14, U15, U18, U21, U23, U65, U71, U80, and U83. Of these, U8 and U18 were IE/regulatory genes, U80 was a replication gene, U12, U21, and U23 encoded glycoproteins, U11 encoded a viral protein, and U83 encoded a chemokine (76). ORFs with 70 to 80% identity were U54, U79, U91, U97, U98, U99, and U100. U79 was a replication gene, U97, U98, U99, and U100 encoded glycoproteins, and U54 was a viral protein gene. ORFs with <70% identity were LT1, DR2, DR7, DR8, LJ1, RJ1, U1, U47, U86, U89, U90, and U95. It is possible that high divergence in amino acid sequence leads to biological and pathogenic differences between variants A and B. Some of these 12 divergent genes could be directly involved in such functional differences. The ORFs with different starting or termination sites were classified as having different sizes if the difference was more than five deduced amino acid residues. The ORFs with different starting sites were DR2, DR7, DR8, LJ1, U1, U2, U3, U7, U8, U21, U23, U47, U55, U83, U98, and U100. The ORFs with different termination sites were DR7, DR8, LJ1, U1, U10, U20, and U47. The ORFs containing insertions were DR2, DR3, DR7, U11, U47, U86, U89, and U95. Finally, in the case of the chemokine receptor (U12) and gp82/105 (U97, HN3, U98, U99, and U100), the differences were due to differential splicing.
(i) IE/regulatory genes.
IE proteins control the temporal cascade of gene expression and are a second line for determining the cellular specificity of infection. Based on in vitro chloramphenicol acetyltransferase or luciferase assays, at least eight genes that might serve as transcriptional activators have been defined: DR7, U3, U16/U17, U18/U19, U27, U86, U89, and U94 (13, 34, 42, 46, 66, 67, 75). There are multiple US22 genes in HCMV, HHV-6, and HHV-7 (5), and this feature appears to be a characteristic of the betaherpesvirus subgroup (7, 16). The translation products of these genes in strain HST differed greatly in length (89 to 1,197 amino acid residues), but they each contained at least one of four US22 amino acid sequence motifs (7, 16). This US22 gene family of HCMV is transcribed with IE kinetics and possesses transactivating functions (57). Strain HST contained 11 ORFs of the US22 gene family: DR1, DR2, DR6, DR7, U2, U3, U7, U8, U16, U25, and U95 (Table 1 and Fig. 2). cDNA analysis showed that HN1 and U90 corresponded to exons of both the IE1 and IE2 genes, which also contained U89 and U86, respectively (18). Amino acid sequence comparisons also suggested that other regions, including U42 and U54, could be involved in transcriptional activation. U42 is a member of the only family of genes involved in transactivation that is conserved among all of the herpesviruses. An HCMV homolog, UL69, has been shown to transactivate gene expression in vitro (71), but studies of the HSV-1 homolog showed that it also has roles in transcriptional termination and in snRNP distribution (37, 51). U54 has similarity to the duplicated genes UL82 and UL83 of HCMV, and UL82 has been shown to be a transcriptional activator and a component of the tegument (29). It is noteworthy that the adeno-associated virus 2 rep gene homolog identified in HHV-6, capable of specifying transregulatory and adeno-associated virus 2 DNA replication functions, was absent in other herpesviruses (67, 68). Among the IE/regulatory genes (DR1, DR2, DR6, DR7, U2, U3, U7, U8, U16, U17, U18, U19, U25, U27, U42, U54, U86/U90, U89/U90, U94, and U95), amino acid sequence comparisons revealed the following amino acid sequence identities between strains HST and U1102: U2, U3, U7, U16, U17, U19, U25, U27, U42, and U94, >90%; DR1, DR6, U8, and U18, 80 to 90%; U54, 79.5%; and DR2, DR7, U86, U89, U90, and U95, <70%.
(ii) Replication genes.
Each herpesvirus that has been sequenced contains a set of conserved replication genes that are essential for viral DNA replication during productive infection. The core replication genes in HHV-6 were those encoding DNA polymerase (U38), DNA polymerase processivity factor (U27), a major DNA binding protein (a single-stranded DNA binding protein; U41), and components of the heterotrimeric helicase/primase complex (U43, U74, and U77). Other genes necessary for DNA replication were specific to herpesvirus subgroups and/or particular viruses. The alphaherpesvirus origin binding protein homolog encoded by HHV-6 (U73) presumably is also functionally analogous (17, 25). The UL84, UL112, and UL113 genes of HCMV likely encode proteins essential for replication functions, such as those enhancing the expression of the replication genes (21). HHV-6 encoded U55, U79, and U80, which were homologous to UL84, UL112, and UL113, respectively (16). Comparison of the deduced amino acid sequences of the replication genes (U27, U38, U41, U43, U55, U73, U74, U77, U79, and U80) and revealed that U27, U38, U41, U43, U55, U73, U74, and U77 showed >90% identity between strains HST and U1102 and that U79 and U80 showed 77.4 and 81.0% identities, respectively. cDNA analysis showed that U80 was an exon of U79, and five different cDNAs were expressed from this region in infected cells, due to alternative splicing (65). All replication genes except U79 and U80 were highly conserved between the two variants. These results suggest that replication genes may not contribute to the biological differences between these two variants.
(iii) Nucleotide metabolism and DNA repair.
We identified HHV-6 homologs of herpesvirus gene products that are involved indirectly in DNA replication by acting as nucleotide substrates or through DNA repair. These gene products included ribonucleotide reductase (U28), dUTPase (U45), phosphotransferase (U69), alkaline exonuclease (U70), and uracil-DNA glycosylase (U81). The ribonucleotide reductase homolog of HHV-6 was encoded by a single ORF specifying a single protein (7, 15, 44), as is the case in the other betaherpesviruses (HHV-7 and HCMV), while the ribonucleotide reductase homolog of alphaherpesvirus, gammaherpesvirus, and cells is formed with subunits. HHV-6 was sensitive to ganciclovir (6, 41), though we do not know which gene(s) is associated with the phosphorylation of ganciclovir. The phosphotransferase gene product of HCMV (UL97), which is a homolog of HHV-6 U69, can phosphorylate nucleotides and the antiviral nucleoside analogue ganciclovir (28, 58). The HHV-6 dUTPase and uracil-DNA glycosylase homologs presumably specify enzymatic activities involved in the excision of uridine residues from DNA, like the U81 gene product, which possesses uracil-DNA glycosylase activity when expressed in E. coli (54). Among HHV-6 ORFs, all of the genes involving nucleotide metabolism and DNA repair (U28, U45, U69, U70, and U81) were strictly conserved, exhibiting more than 94% identity between strains HST and U1102. These results suggest that nucleotide metabolism and DNA replication genes may not be involved in the functional divergence between variants A and B.
(iv) Glycoproteins.
In all sequenced herpesviruses, there are conserved glycoprotein genes encoding glycoprotein B (gB), gH, gM, and gL, all of which were also conserved in HHV-6 (U39, U48, U72, and U82, respectively). gB and gH are structurally highly conserved between herpesviruses, are membrane bound, and appear to play roles in virus-cell fusion and cellular spread of virus infection. Both gB and gH of HHV-7 are encoded by late genes (56), whereas gB and gH of HHV-6 are encoded by IE and early-late genes, respectively (19, 39). However, the actual translation of the gB gene of HHV-6B occurs at the late phase (63). These results suggest that the gB gene of HHV-6 is regulated posttranscriptionally. gL physically associates with the gH precursor protein and may be required for gH transport and/or processing (30, 31). gM is fairly conserved between herpesviruses and was shown to be a component of the virus particle in HSV-1 and equine herpesvirus type 1 (4, 52).
HHV-6 contained two genes (U12 and U51) encoding G-protein-coupled receptor homologs. U12 functionally encodes a calcium-mobilizing receptor for the β-chemokines RANTES, MIP-1α and -1β, and MCP-1 but not for the α-chemokine interleukin-8 (19). HHV-6 U51 was a positional and structural homolog of the HHV-7 and HCMV UL78, showing relatively high sequence similarity to the G-protein-coupled receptor of herpesvirus saimiri (15, 45). This similarity could be an indication that they were derived from the same gene within a progenitor herpesvirus genome. Interestingly, the HHV-6 U51 gene product showed the closest sequence similarity to a cellular opioid receptor.
A highly spliced gene at the right end of the UR of the genome has been characterized by analyzing a cDNA resulting from multiple splicing, and it encodes the glycoprotein gp82/105 (50). In the case of strain GS, this gene contains 12 exons, and the single long ORF begins within exon 3 and ends in exon 12. In contrast, in strain HST, this gene contained eight or nine exons, and the single long ORF began within exon 2 and ended in exon 8 (61). The single long ORF encoding gp82/105 in strain GS is longer than in strain HST. The differential splicing may play a role in the generation of various mRNA species that encode some protein components in the gp82/105 complex of the two variants. Furthermore, the amino acid sequence of each exon was divergent between variants A and B (Table 1), and some monoclonal antibodies for gp82/105 are variant A-specific neutralizing antibodies (49). Taken together, these findings suggest that the high degree of divergence in gp82/105 may lead to differences in the cell tropism between variants A and B.
HHV-6 ORFs U20, U21, U23, U24, and U85 were predicted to encode glycoproteins. U20 and U85 encode homologs of the immunoglobulin E (IgE) C chain and OX-2 membrane antigen, both of which are members of the Ig superfamily (10, 16). U24 of strain HST lacked an N-terminal signal sequence and may represent an exon (15).
Genes encoding glycoproteins were U12, U20, U21, U22, U23, U39, U48, U51, U72, U82, U85, U97, HN2, U98, U99, and U100. Among these, the ORFs with >90% identity were U20, U22, U39, U48, U51, U72, U82, and U85; ORFs with 80 to 90% identity were U12, U21, and U23; and those with 70 to 80% identity were U97, U98, U99, and U100. Of these glycoproteins, gp82/105 exhibited the most divergence.
(v) Capsid, tegument, and virus assembly proteins.
Several ORFs of HHV-6 were homologous to herpesvirus genes encoding characterized or candidate structural proteins. Table 1 shows that these include homologs of the major capsid protein (U57), minor capsid protein (U29), large tegument protein (U31), and virion proteins (U33, U34, U36, U50, U56, and U76) (7, 15, 36, 44). Betaherpesvirus-specific and conserved structural proteins are encoded by HHV-6 U11 (major antigenic phosphoprotein, pp100) (47, 48) and U54 (tegument transactivator), which are homologs of HCMV UL32 (antigenic phosphoprotein, pp150) and UL82/U83 (tegument transactivator, pp65/72K), respectively. The gene products involved in DNA packaging and capsid assembly corresponded to HHV-6 U29, U30, U53, and U60/U66. The U53 gene encoded the protease/assembly protein (assemblin) and the scaffolding protein. Assemblin and scaffolding protein are derived from proteolytic cleavage and internal initiation (15, 59, 69).
The genes U11, U29, U30, U31, U33, U34, U36, U49, U50, U53, U54, U56, U57, U60/U66, and U76 encoded viral structural proteins, and all except U11 and U54 showed >90% identity between strains HST and U1102. In contrast, U11, a viral protein, and U54, a tegument transactivator, showed rather low identity (80.1 and 79.5%, respectively). These results suggest that the replication genes, except those for the U11 viral protein and the U54 tegument transactivator, may not be involved in the functional differences between variants A and B.
Major repetitive elements.
It has been observed that a remarkable divergence in the nucleotide sequence between strains JI and RK is present only in the DR (38, 44). Similarly, between strains HST and U1102, marked differences were observed only in specific regions of the DR and the junction between the UR and DR. In the DRs of both HST and U1102, the basic element of regions T1 and T2 (TAACCC) was related to human telomeric sequences (Fig. 2). These reiterations consisted of arrays of the basic element interspersed with 11 types of human telomere-related elements, all hexonucleotides. In the case of strain HST, T1 was the longer reiteration, consisting of 54 copies of the telomeric element: 26 copies of the basic telomeric element and 28 copies of the human telomere-related elements. In contrast, T2 consisted of 26 copies of the basic telomeric element alone. In the case of strain U1102, T1 consisted of 54 copies of the telomeric element: 14 copies of the basic telomeric element and 40 copies of the related telomeric elements, most of which were also hexonucleotides but some of which were larger. Furthermore, T2 of U1102 consisted of 59 copies of the basic element alone. Thus, T2 of HST was half the size of that of U1102, and T1 of HST contained more conserved telomeric elements than that of U1102.
Repeat region R1 contains 52 copies of a dodecameric repeat element, consisting of its basic element (GAGGCCCTGCTG) and variants. However, it was difficult to determine the copy number of the dodecameric repeat element in R1 of U1102, because the reiterations in R1 consist of remarkably variant elements. R1 was located in U86, the putative IE2 locus of HHV-6. R2 consisted of two types of repeats, a 79- to 80-bp-long tandem repeat and a G/T repeat. The tandem repeat region in R2 of strain HST contained five copies of a 79- to 80-bp repeat element, while that of U1102 contained two copies of imperfect repeats. R3 (Kpn repeat region) contained 24 copies of tandemly repeated 104- to 107-bp-long elements and 10 recognition sites for KpnI in strain HST (24), while R3 of U1102 contained 28 copies of tandemly repeated 104- to 107-bp-long elements, all of which contained recognition sites for KpnI. Because of these differences, the region corresponding to the U92- and U93-homologous ORFs in strain HST was divided by six small ORFs and a small part of HN2. On the whole, the repeat elements of strain HST were more highly conserved than those of strain U1102.
Genetic divergence between HHV-6 variants.
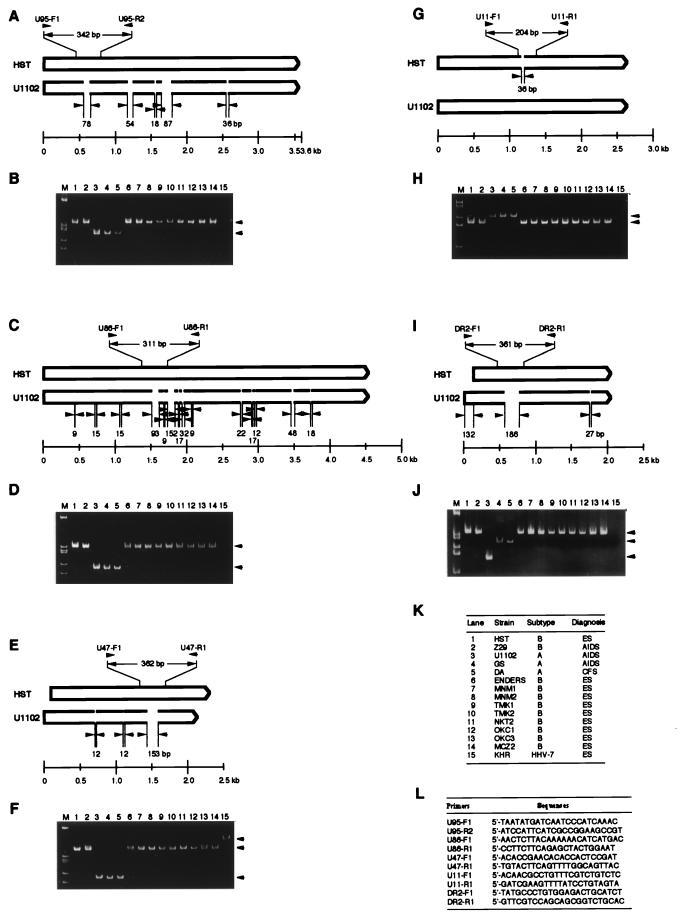
The marked divergence of variants A and B permitted the design of variant-specific oligonucleotide primers to detect DNA fragments that differed in size between variants A and B HHV-6 DNA using PCR. To analyze the genetic polymorphism of HHV-6 isolates, we examined 14 different strains belonging to HHV-6 and one HHV-7 strain, KHR (Fig. 3K). In this experiment, viral DNAs were extracted from human umbilical CBMCs that were infected with each virus using the protease K treatment method. PCR was performed as described elsewhere (19), using the appropriate pairs of primers for U95, U86, U47, U11, and DR2, as shown in Fig. 3. No positive PCR products were detected with uninfected cells or cells infected with other human herpesviruses, including HSV-1 and -2, varicella-zoster virus, HCMV, and Epstein-Barr virus (data not shown). In the case of HHV-7 (Fig. 3B, D, F, H, and J, lanes 15), however, among the genes tested, a positive PCR product was detected only for the U47 gene, which was larger than those of HHV-6 (Fig. 3F). HHV-6 DNA was detected in all 14 isolates by this method, and PCR products were clearly separated into two groups in the case of the U95, U86, U47, and U11 genes: variants B and A showed fragments of 342, 311, 362, and 168 bp and of 264, 209, 209, and 204 bp, respectively (Fig. 3K; also see Fig. 3B, D, F, and H). After amplification of the DR2 genes, however, PCR products were clearly separated into three groups: 361 bp (variant B), 175 bp (U1102), and approximately 265 bp (GS and DA) (Fig. 3J). These differences observed in variant A may be associated with the characteristic divergence of variant A viruses.
FIG. 3.
PCR typing of HHV-6. (A, C, E, G, and I) Schematic primary structures of ORFs U95, U86, U47, U11, and DR2 of strains HST and U1102. Positions and directions of the PCR primers are indicated by arrowheads at vertical lines. (B, D, F, H, and J) PCR amplification of DNAs from isolates 1 to 15 (K) of HHV-6 and HHV-7. Templates were purified from peripheral blood mononuclear cells infected with several laboratory strains and clinical isolates and were amplified with PCR primers (L). The products were fractionated by electrophoresis on 6% polyacrylamide gels and stained with ethidium bromide. The PCR products are indicated by arrowheads in panels B, D, F, H, and J. (K) Viruses used in the experiment. CFS, chronic fatigue syndrome.
DISCUSSION
We have determined the complete DNA sequence of HHV-6 variant B strain HST, the causative agent of ES, and identified 115 potential ORFs within the 161,573-bp contiguous sequence of the entire HHV-6 genome. When the sequence was compared with that of the variant A strain U1102, some genes with remarkable differences in amino acid identity were identified.
We conclude that the LT1, DR3, DR8, LJ1, RJ1, and DR3′ ORFs probably do not encode proteins because LT1 and DR3 did not contain poly(A) signals, the ORFs of DR8, LJ1, and RJ1 contained telomeric sequences, and DR3′ contained a telomeric sequence between the ORF and the poly(A) signal. As variant A-specific ORFs DR4, DR5, U78, U88, U92, and U93 were small and did not exhibit homology with HHV-6B, HHV-7, other herpesvirus, and/or cellular genes, it is not likely that these genes encode functional proteins either. In contrast, HN1 and HN3 were variant B-specific genes and corresponded to exons of the IE1/IE2 and gp82/105 genes, respectively. We do not know whether HN2, DRHN1, and DRHN2 have homologous cellular genes or encode functional proteins.
All of the ORFs with <70% identity between the two variants were found to include deleted regions when the above-mentioned ORFs were excluded from the comparison (Fig. 3 and reference 73). The remarkable differences except for U47 accumulated in some of the IE/regulatory genes (DR2, DR7, U86/90, U89/90, and U95) and may lead to characteristic differences between variants A and B, although point mutations that we have not detected could be associated with these differences. The variation in IE proteins may be relevant to variant divergence because IE proteins control the temporal cascade of gene expression and are a second line in determining the cellular specificity of infection. Chou and Marousek (9) reported that variant B isolates segregate into two groups with even less divergence between them (92.6% identity). U89 derived from clinical isolates and strain Z29, which belongs to group 1, show more than 99.4% amino acid identity. In contrast, U89 of strain HST had 92.3% amino acid identity compared with strain Z29 (Table 1). Clinical isolates, which belong to group 2, have more than 99.4% amino acid identity compared to strain HST. These results suggest that strain HST belongs to group 2, although we do not know what function this divergence is associated with. In the case of U47, we cannot predict its function, but homologous genes exist in HHV-7 and HCMV (U47 and UL74, respectively).
HHV-6 genes transcribed under IE conditions, that is, in the absence of prior protein synthesis, are U16/U17, U39, U42, U81, U89/U90, U91, and U94 ORFs (39). As some of these transcripts are not translated under IE conditions, they may be regulated in the posttranscriptional phase. If this is the case, some regulatory gene(s) likely plays an important role, and this mechanism would be quite different from the known transcriptional regulation of the herpesviruses.
In all sequenced herpesviruses, conserved glycoprotein genes encode gB, gH, gM, and gL, and the existence of neutralizing antibodies for gB and gH suggests that gB and gH/gL are essential for infection (62, 64). HSV-1 encodes an additional glycoprotein, gD, which recognizes cellular receptors (HveA, HveB, and HveC) (14, 40, 70), while HHV-6 encodes a different additional glycoprotein, gp82/105, which is recognized by neutralizing antibodies (49). This finding suggests that gp82/105 may recognize a cellular receptor(s). In addition, the gp82/105 glycoprotein of HHV-6 showed the highest divergence in amino acid sequence between the two variants. It is possible that gp82/105 leads to the difference of cell tropism between variants A and B.
U83 of strain HST encodes a chemokine which functions as a chemoattractant for monocytes (76). Similarly, U83 of strain U1102 encodes a chemokine homolog, but it does not contain a signal peptide sequence. Thus, U83 of strain U1102 may not be secreted and thus cannot exert its chemotactic activity. If U83 functions as a chemoattractant in vivo, it is possible that HHV-6 can easily spread through cell-to-cell infection, even in the presence of neutral antibodies. Although we do not know whether the U83 gene product of HHV-6A contains a signal peptide, if it does not, we predict that variant B viruses could easily spread in blood, whereas variant A viruses should not.
In analyzing the divergence in the functional proteins of HHV-6A and HHV-6B, we must consider that splicing would affect the gene layout and the level of relatedness between the HHV-6A and HHV-6B proteins. We have already obtained the splicing patterns of some mRNAs. These patterns are not as simple as predicted by Megaw et al. (38). For example, in the case of U79 and U80, there are a few more donor and acceptor splice sites than predicted by Megaw et al., and at least five kinds of mRNAs encoded by them can be generated by alternative splicing (65). In the case of IE1 and IE2, U90 is linked to U89 or U86 by alternative splicing. In the case of gp82/105, although there are donor and acceptor motifs, some signals on the mRNA appear not to be functional (50, 61). We currently have no information about splicing in HHV-6A, except in the case of gp82/105. Further studies of splicing patterns showing functional proteins may provide new insights into biological divergence between HHV-6A and HHV-6B and molecular analysis of the proteins.
Finally, we would like to point out that the divergence we have observed with IE/regulatory genes, gp82/105, and U83 between variants A and B may lead to biological and pathogenic differences between the two variants. Further functional studies of the genes showing such remarkable divergence between variants may provide new insights into mechanisms for the molecular pathogenesis and cell tropism of HHV-6 infection.
ACKNOWLEDGMENTS
We are grateful to P. L. Ward for critical reading of and valuable comments on the manuscript.
This study was partly supported by a grant-in-aid for general scientific research from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Ablashi D V, Balachandran N, Josephs S F, Hung C L, Krueger G R, Kramarsky B, Salahuddin S Z, Gallo R C. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology. 1991;184:545–552. doi: 10.1016/0042-6822(91)90424-a. [DOI] [PubMed] [Google Scholar]
- 2.Aubin J T, Collandre H, Candotti D, Ingrand D, Rouzioux C, Burgard M, Richard S, Huraux J M, Agut H. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J Clin Microbiol. 1991;29:367–372. doi: 10.1128/jcm.29.2.367-372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubin J T, Agut H, Collandre H, Yamanishi K, Chandran B, Montagnier L, Huraux J M. Antigenic and genetic differentiation of the two putative types of human herpes virus 6. J Virol Methods. 1993;41:223–234. doi: 10.1016/0166-0934(93)90129-f. [DOI] [PubMed] [Google Scholar]
- 4.Baines J D, Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993;67:1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berneman Z N, Ablashi D V, Li G, Eger-Fletcher M, Reitz M S, Hung C L, Brus I, Komaroff A L, Gallo R C. Human herpesvirus 7 is a T-lymphotrophic virus and is related to, but significantly different from, human herpesvirus 6 and human cytomegalovirus. Proc Natl Acad Sci USA. 1992;89:10552–10556. doi: 10.1073/pnas.89.21.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns W H, Sandford G R. Susceptibility of human herpesvirus 6 to antivirals in vitro. J Infect Dis. 1990;162:634–637. doi: 10.1093/infdis/162.3.634. [DOI] [PubMed] [Google Scholar]
- 7.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchinson C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. Analysis of the protein coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 8.Chandran B, Tirawatnapong S, Pfeiffer B, Ablashi D V. Antigenic relationships among human herpesvirus-6 isolations. J Med Virol. 1992;37:247–254. doi: 10.1002/jmv.1890370403. [DOI] [PubMed] [Google Scholar]
- 9.Chou S, Marousek G I. Analysis of interstrain variation in a putative immediate-early region of human herpesvirus 6 DNA and definition of variant-specific sequences. Virology. 1994;198:370–376. doi: 10.1006/viro.1994.1044. [DOI] [PubMed] [Google Scholar]
- 10.Clark M J, Gagnon J, Williams A F, Barklay A N. MRC OX-2 antigen: a lymphoid/neuronal membrane glycoprotein with a structure like a single immunoglobulin light chain. EMBO J. 1985;4:113–118. doi: 10.1002/j.1460-2075.1985.tb02324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downing R G, Sewankambo N, Serwadda D, Honess R, Crawford D, Jaret R, Griffin B E. Isolation of human lymphotropic herpesvirus from Uganda. Lancet. 1987;ii:390. doi: 10.1016/s0140-6736(87)92403-2. [DOI] [PubMed] [Google Scholar]
- 12.Enders G, Biber M, Meyer G, Helftenbein E. Prevalence of antibodies to human herpesvirus 6 in different age groups, in children with exanthema subitum, other acute exanthematous childhood diseases, Kawasaki syndrome, and acute infections with other herpesvirus and HIV. Infection. 1990;18:12–15. doi: 10.1007/BF01644173. [DOI] [PubMed] [Google Scholar]
- 13.Geng Y, Chandran B, Josephs S F, Wood C. Identification and characterization of a human herpesvirus 6 gene segment that trans activates the human immunodeficiency virus type 1 promoter. J Virol. 1992;66:1564–1570. doi: 10.1128/jvi.66.3.1564-1570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 15.Gompels U A, Carrigan D R, Carss A L, Arno J. Two groups of human herpesvirus 6 identified by sequence analyses of laboratory strains and variants from Hodgkin’s lymphoma and bone marrow transplant patients. J Gen Virol. 1993;74:613–622. doi: 10.1099/0022-1317-74-4-613. [DOI] [PubMed] [Google Scholar]
- 16.Gompels U A, Nicholas J, Lawrence G, Jones M, Thomson B J, Martin M E, Efstathiou S, Craxton M, Macaulay H A. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 17.Inoue N, Dambaugh T R, Rapp J C, Pellett P E. Alphaherpesvirus origin-binding protein homolog encoded by human herpesvirus 6B, a betaherpesvirus, binds to nucleotide sequences that are similar to ori regions of alphaherpesvirus. J Virol. 1994;68:4126–4136. doi: 10.1128/jvi.68.7.4126-4136.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isegawa, Y., et al. Unpublished data.
- 19.Isegawa Y, Ping Z, Nakano K, Sugimoto N, Yamanishi K. Human herpesvirus 6 open reading frame U12 encodes a functional β-chemokine receptor. J Virol. 1998;72:6104–6112. doi: 10.1128/jvi.72.7.6104-6112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isegawa Y, Sheng J, Sokawa Y, Yamanishi K, Nakagomi O, Ueda S. Selective amplification of cDNA sequence from total RNA by cassette-ligation mediated polymerase chain reaction (PCR): application to sequencing 6.5 kb genome segment of hantavirus strain B-1. Mol Cell Probes. 1992;6:467–475. doi: 10.1016/0890-8508(92)90043-w. [DOI] [PubMed] [Google Scholar]
- 21.Iskenderian A C, Huang L, Reilly A, Stenberg R M, Anders D G. Four of eleven loci required for transient complementation of human cytomegalovirus DNA replication cooperate to activate expression of replication genes. J Virol. 1996;70:383–392. doi: 10.1128/jvi.70.1.383-392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josephs S F, Salahuddin S Z, Ablashi D V, Schachter F, Wong-Staal F, Gallo R C. Genomic analyses of the human B lymphotropic virus. Science. 1986;234:601–603. doi: 10.1126/science.3020691. [DOI] [PubMed] [Google Scholar]
- 23.Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol. 1991;72:1401–1408. doi: 10.1099/0022-1317-72-6-1401. [DOI] [PubMed] [Google Scholar]
- 24.Kosuge H, Isegawa Y, Yamanishi K. Nucleotide sequence analysis of a 30-kilobase-pair region of human herpesvirus-6B (HHV-6B) genome and strain-specific variations in major immediate-early genes. Virus Res. 1997;52:1–14. doi: 10.1016/s0168-1702(97)00099-3. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence G L, Nicholas J, Barrell B G. Human herpesvirus 6 (strain U1102) encodes homologues of the conserved herpesvirus glycoprotein gM and the alphaherpesvirus origin-binding protein. J Gen Virol. 1995;76:147–152. doi: 10.1099/0022-1317-76-1-147. [DOI] [PubMed] [Google Scholar]
- 26.Lindquester G J, Greenamoyer C A, Anton E D, O’Brian J J, Pellett P E, Dambaugh T R. Comparison of a 20 kb region of human herpesvirus 6B with other human beta herpesviruses reveals conserved replication genes and adjacent divergent open reading frames. Arch Virol. 1997;142:193–204. doi: 10.1007/s007050050070. [DOI] [PubMed] [Google Scholar]
- 27.Lindquester G J, O’Brian J J, Anton E D, Greenamoyer C A, Pellett P E, Dambaugh T R. Genetic content of a 20.9 kb segment of human herpesvirus 6B strain Z29 spanning the homologs of human herpesvirus 6A genes U40-57 and containing the origin of DNA replication. Arch Virol. 1997;142:103–123. doi: 10.1007/s007050050062. [DOI] [PubMed] [Google Scholar]
- 28.Littler E, Stuart A D, Chee M S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the anti-viral nucleoside analogue ganciclovir. Nature (London) 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Stinski M F. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J Virol. 1992;66:4434–4444. doi: 10.1128/jvi.66.7.4434-4444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D X, Gompels U A, Foa-Tomasi L, Campadelli-Fiume G. Human herpesvirus-6 glycoprotein H and L homologs are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology. 1993;197:12–22. doi: 10.1006/viro.1993.1562. [DOI] [PubMed] [Google Scholar]
- 31.Liu D X, Gompels U A, Nicholas J, Lelliot G. Identification and expression of the human herpesvirus-6 glycoprotein H and interaction with an accessory 30 kilodalton glycoprotein. J Gen Virol. 1993;74:1847–1857. doi: 10.1099/0022-1317-74-9-1847. [DOI] [PubMed] [Google Scholar]
- 32.Lopetz C, Pellett P, Stewart J, Goldsmith C, Sanderlin K, Black J, Warfield D, Feorino P. Characteristics of human herpesvirus-6. J Infect Dis. 1988;157:1271–1273. doi: 10.1093/infdis/157.6.1271. [DOI] [PubMed] [Google Scholar]
- 33.Lusso P, Markham P D, Tschachler E, di Marzo Veronese F, Salahuddin S Z, Ablashi D V, Pahwa S, Krohn K, Gallo R C. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6) J Exp Med. 1988;167:1659–1670. doi: 10.1084/jem.167.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin M E D, Nicholas J, Thomson B J, Newman C, Honess R W. Identification of a transactivating function mapping to the putative immediate-early locus of human herpesvirus 6. J Virol. 1991;65:5381–5390. doi: 10.1128/jvi.65.10.5381-5390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin M E, Thomson B J, Honess R W, Craxton M A, Gompels U A, Liu M Y, Littler E, Arrand J R, Teo I, Jones M D. The genome of human herpesvirus 6: maps of unit-length and concatemeric genomes for nine restriction endonucleases. J Gen Virol. 1991;72:157–168. doi: 10.1099/0022-1317-72-1-157. [DOI] [PubMed] [Google Scholar]
- 36.McGeoch D J. The genomes of the human herpesviruses: contents, relationships, and evolution. Annu Rev Microbiol. 1989;43:235–265. doi: 10.1146/annurev.mi.43.100189.001315. [DOI] [PubMed] [Google Scholar]
- 37.McLauchlan J, Phelan A, Loney C, Sandri-Goldin R M, Clements J B. Herpes simplex virus IE63 acts at the posttranscriptional level stimulates viral mRNA 3′ processing. J Virol. 1992;66:6939–6945. doi: 10.1128/jvi.66.12.6939-6945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Megaw A G, Rapaport D, Avidor B, Frenkel N, Davison A J. The DNA sequence of the RK strain of human herpesvirus 7. Virology. 1998;244:119–132. doi: 10.1006/viro.1998.9105. [DOI] [PubMed] [Google Scholar]
- 39.Mirandola P, Menegazzi P, Merighi S, Ravaioli T, Cassai E, Di Luca D. Temporal mapping of transcripts in herpesvirus 6 variants. J Virol. 1998;72:3837–3844. doi: 10.1128/jvi.72.5.3837-3844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 41.Mookerjee B P, Vogelsang G. Human herpes virus-6 encephalitis after bone marrow transplantation: successful treatment with ganciclovir. Bone Marrow Transplant. 1997;20:905–906. doi: 10.1038/sj.bmt.1700988. [DOI] [PubMed] [Google Scholar]
- 42.Mori Y, Yagi H, Shimamoto T, Isegawa Y, Sunagawa T, Inagi R, Kondo K, Tano Y, Yamanishi K. Analysis of human herpesvirus 6 U3 gene, which is a positional homolog of human cytomegalovirus UL24 gene. Virology. 1998;249:129–139. doi: 10.1006/viro.1998.9305. [DOI] [PubMed] [Google Scholar]
- 43.Mukai T, Yamamoto T, Kondo T, Kondo K, Okuno T, Kosuge H, Yamanishi K. Molecular epidemiological studies of human herpesvirus 6 in families. J Med Virol. 1994;42:224–227. doi: 10.1002/jmv.1890420303. [DOI] [PubMed] [Google Scholar]
- 44.Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J Virol. 1996;70:5975–5989. doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholas J, Cameron K R, Honess R W. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature (London) 1992;355:362–365. doi: 10.1038/355362a0. [DOI] [PubMed] [Google Scholar]
- 46.Nicholas J, Martin M. Nucleotide sequence analysis of a 38.5-kilobase-pair region of the genome of human herpesvirus 6 encoding human cytomegalovirus immediate-early gene homologs and transactivating functions. J Virol. 1994;68:597–610. doi: 10.1128/jvi.68.2.597-610.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nipel F, Ellinger K, Fleckenstein B. Gene for the major antigenic structural protein (p100) of human herpesvirus 6. J Virol. 1992;66:3918–3924. doi: 10.1128/jvi.66.6.3918-3924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pellett P E, Sanchez-Martinez D, Dominguez G, Black J B, Anton E, Greenamoyer C, Dambaugh T R. A strongly immunoreactive virion protein of human herpesvirus 6 variant B strain Z29: identification and characterization of the gene and mapping of a variant-specific monoclonal antibody reactive epitope. Virology. 1993;195:521–531. doi: 10.1006/viro.1993.1403. [DOI] [PubMed] [Google Scholar]
- 49.Pfeiffer B, Berneman Z N, Neipel F, Chang C K, Tirwatnapong S, Chandran B. Identification and mapping of the gene encoding the glycoprotein complex gp82-gp105 of human herpesvirus 6 and mapping of the neutralizing epitope recognized by monoclonal antibodies. J Virol. 1993;67:4611–4620. doi: 10.1128/jvi.67.8.4611-4620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfeiffer B, Thomson B, Chandran B. Identification and characterization of a cDNA derived from multiple splicing that encodes envelope glycoprotein gp105 of human herpesvirus 6. J Virol. 1995;69:3490–3500. doi: 10.1128/jvi.69.6.3490-3500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phelan A, Carmo-Fonseca M, McLauchlan J, Lamond A I, Clements J B. A herpes simplex virus type 1 immediate-early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc Natl Acad Sci USA. 1993;90:9056–9060. doi: 10.1073/pnas.90.19.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pilling A, Davison A J, Telford E, Meredith D M. The equine herpesvirus type 1 glycoprotein M is a major constitute of the virus particle. J Gen Virol. 1994;75:439–442. doi: 10.1099/0022-1317-75-2-439. [DOI] [PubMed] [Google Scholar]
- 53.Salahuddin S Z, Ablashi D V, Markham P, Josephs S F, Sturzegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, Gallo R C. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234:596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 54.Sato S, Yamamoto T, Isegawa Y, Yamanishi K. Identification of human herpesvirus 6 uracil-DNA glycosylase gene. J Gen Virol. 1994;75:2349–2354. doi: 10.1099/0022-1317-75-9-2349. [DOI] [PubMed] [Google Scholar]
- 55.Schirmer E C, Wyatt L S, Yamanishi K, Rodriguez W J, Frenkel N. Differentiation between two distinct classes of viruses now classified as human herpesvirus 6. Proc Natl Acad Sci USA. 1991;88:5922–5926. doi: 10.1073/pnas.88.13.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Secchiero P, Berneman Z N, Sun D, Nichlas J, Reitz M S., Jr Identification of envelope glycoproteins H and B homologous of human herpesvirus 7. Intervirology. 1997;40:22–32. doi: 10.1159/000150517. [DOI] [PubMed] [Google Scholar]
- 57.Stasiak P C, Mocarski E S. Transactivation of the cytomegalovirus ICP36 gene promoter requires the α gene product TRS1 in addition to IE1 and IE2. J Virol. 1992;66:1050–1058. doi: 10.1128/jvi.66.2.1050-1058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan V, Talarico C L, Stanat S C, Davis M, Coen D M, Biron K K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalo-infected cells. Nature (London) 1992;358:162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- 59.Sunagawa, T., et al. Unpublished data.
- 60.Takahashi K, Sonoda S, Higashi K, Kondo T, Takahashi H, Takahashi M, Yamanishi K. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J Virol. 1989;63:3161–3163. doi: 10.1128/jvi.63.7.3161-3163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takeda, K., et al. Unpublished data.
- 62.Takeda K, Haque M, Sunagawa T, Okuno T, Isegawa Y, Yamanishi K. Identification of a variant B-specific neutralization epitope on glycoprotein H of human herpesvirus-6. J Gen Virol. 1997;78:2171–2178. doi: 10.1099/0022-1317-78-9-2171. [DOI] [PubMed] [Google Scholar]
- 63.Takeda K, Nakagawa N, Yamamoto T, Inagi R, Kawanishi K, Isegawa Y, Yamanishi K. Prokaryotic expression of an immediate-early gene of human herpesvirus 6 and analysis of its viral antigen expression in human cells. Virus Res. 1996;41:193–200. doi: 10.1016/0168-1702(96)01287-7. [DOI] [PubMed] [Google Scholar]
- 64.Takeda K, Okuno T, Isegawa Y, Yamanishi K. Identification of a variant A-specific neutralizing epitope on glycoprotein B (gB) of human herpesvirus-6 (HHV-6) Virology. 1996;222:176–183. doi: 10.1006/viro.1996.0408. [DOI] [PubMed] [Google Scholar]
- 65.Taniguchi, T., et al. Unpublished data.
- 66.Thompson J, Choudhury S, Kashanchi F, Doniger J, Berneman Z, Frenkel N, Rosenthal L J. A transforming fragment within the direct repeat region of human herpesvirus type 6 that transactivates HIV-1. Oncogene. 1994;9:1167–1175. [PubMed] [Google Scholar]
- 67.Thomson B J, Weindler F W, Gray D, Schwaab V, Heilbronn R. Human herpesvirus 6 (HHV-6) is a helper virus for adeno-associated virus type 2 (AAV-2) and the AAV-2 rep gene homologue in HHV-6 can mediate AAV-2 DNA replication and regulate gene expression. Virology. 1994;204:304–311. doi: 10.1006/viro.1994.1535. [DOI] [PubMed] [Google Scholar]
- 68.Thomson B J, Efstathiou S, Honess R W. Acquisition of the human adeno-associated virus type-2 rep gene by human herpesvirus type-6. Nature (London) 1991;351:78–80. doi: 10.1038/351078a0. [DOI] [PubMed] [Google Scholar]
- 69.Tigue N J, Matharu P J, Roberts N A, Mills J S, Kay J, Jupp R. Cloning, expression, and characterization of the proteinase from human herpesvirus 6. J Virol. 1996;70:4136–4141. doi: 10.1128/jvi.70.6.4136-4141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 71.Winkler M, Rice S A, Stamminger T. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J Virol. 1994;68:3942–3954. doi: 10.1128/jvi.68.6.3943-3954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyatt L S, Balachandran N, Frenkel N. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J Infect Dis. 1990;162:852–857. doi: 10.1093/infdis/162.4.852. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto T, Mukai T, Kondo K, Yamanishi K. Variation of DNA sequence in immediate-early gene of human herpesvirus 6 and variant identification by PCR. J Clin Microbiol. 1994;32:473–476. doi: 10.1128/jcm.32.2.473-476.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthema subitum. Lancet. 1988;i:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 75.Zhou Y, Chang C K, Qian G, Chandran B, Wood C. Trans-activation of the HIV promoter by a cDNA and its genomic clones of human herpesvirus-6. Virology. 1994;199:311–322. doi: 10.1006/viro.1994.1129. [DOI] [PubMed] [Google Scholar]
- 76.Zou P, Isegawa Y, Nakano K, Haque M, Horiguchi Y, Yamanishi K. Human herpesvirus 6 open reading frame U83 encodes a functional chemokine. J Virol. 1999;73:5926–5933. doi: 10.1128/jvi.73.7.5926-5933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]