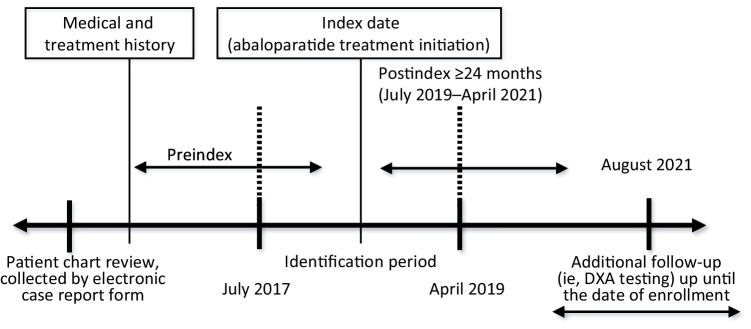
Fig. 2.
Study design. Patient data collected from electronic case report form (eCRF) were reviewed for medical and treatment history during the preindex period. The index date refers to the date of abaloparatide treatment initiation, which occurred during the identification period between July 2017 and April 2019. The postindex period was a period of ≥ 24 months from the date of abaloparatide treatment initiation, between July 2019 and April 2021. Additional follow-up (i.e., DXA testing) occurred up until the date of study enrollment