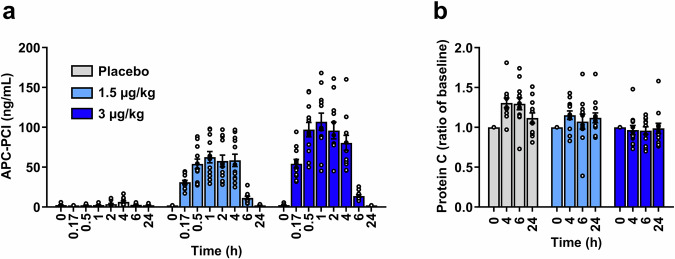
Fig. 3. Pharmacodynamic evaluation of AB002 drug exposure.
Patients were administered a single dose of AB002 at 1.5 µg/kg or 3 µg/kg, or placebo. The surrogate marker of drug exposure, APC-PCI (a), was evaluated in patient plasma samples on study day 1 through 24 h post-dose. Circulating protein C levels (b) were evaluated on study day 1 through 24 h post-dose. Data were normalized to pre-dose values as a ratio of baseline. n = 12 for placebo (gray bars), n = 12 for 1.5 µg/kg (light blue bars), and n = 12 for 3 µg/kg (dark blue bars); data represent means ± SEM. Exact p-values are provided in Supplementary Data 1.