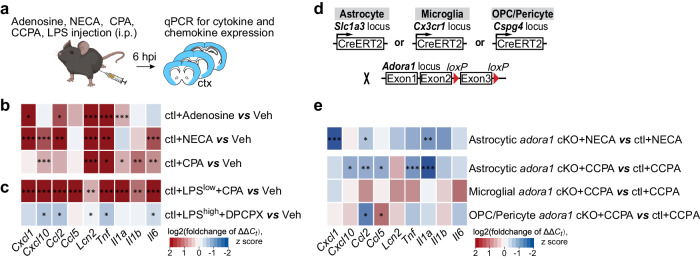
Fig. 2. Peripheral adenosine administration evokes upregulation of inflammation-related genes in the brain.
a Schematic illustration of adenosine, adenosine analogue (NECA), A1 adenosine receptor (A1AR) agonists (CPA, CCPA), and A1AR antagonist (DPCPX) administration experiments. b Expression of inflammation-related genes was enhanced in the mouse cortex six hours post adenosine, NECA, and CPA injections (n = 3 mice per group). c CPA further upregulated the inflammation-related genes in the cortex induced by a peripheral LPSlow (1 mg/kg, i.p.) injection. (n = 3 mice per group). However, DPCPX administration reduced the inflammation-related genes in the cortex induced by a peripheral LPShigh (5 mg/kg, i.p.) injection. (n = 3 mice per group). d Schematic illustration of mouse breeding. e Inflammation-related gene expressions were reduced in the cortex of astrocytic Adora1 cKO mice at 6 hours post NECA (n = 3 mice per group) and CCPA (n = 6 mice per group) injection compared to ctl mice, which was not observed in mice with specific ablation of Adora1 in microglia (using Cx3CR1-CreERT2 mice) and oligodendrocyte precursor cells/pericytes (using NG2-CreERT2 mice, only Ccl2 was reduced while Ccl5 increased). Summary data are presented as the log2(foldchange of ΔΔCt). Statistical significance of each gene expression in b, c, e was assessed to ctl+veh using two tailed unpaired Student’s t test. Bar graphs of b, c, e was presented in Supplementary Fig. 2a–i. Source data and exact P values are provided in the Source Data file. Panel a was created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.