Abstract
Although the impact of group dynamics on creativity is widely recognized, prior research has primarily concentrated on individuals in isolation from social context. To address this lacuna, we focus on groups as the fundamental unit of analysis. We used functional near-infrared spectroscopy (fNIRS) to examine brain activity in groups of four during brainstorming discussions. We assessed interbrain coupling in the dorsolateral prefrontal cortex (DLPFC), a brain region linked to flexibility, and in the inferior frontal gyrus (IFG), a region associated with imitation. Our findings demonstrate that creativity-focused discussions induced interbrain coupling both in regions related to flexibility and herding. Notably, interbrain coupling in the IFG was associated with more imitation of responses. Critically, while interbrain coupling in the DLPFC positively predicted group creativity, in the IFG it negatively predicted creativity. These findings suggest that increase in group mindsets of flexibility relative to herding is important for enhancing group creativity.
Subject terms: Cooperation, Social behaviour
An fNIRS study examining brain activity during group creativity suggests that interbrain coupling in the dorsolateral prefrontal cortex predicts group creativity, while interbrain coupling in the inferior frontal gyrus predicts herding.
Introduction
Humans are keen consumers of creativity. From cavemen paintings and hunter-gatherer toolmaking to contemporary arts and technology, the tendency to create new ideas and products out of otherwise disconnected elements is deeply rooted in human cognition and has contributed to immeasurable human accomplishments1. Whereas creativity is often seen as an individual capacity, scholars are increasingly acknowledging that it is also a social phenomenon that defines various societies2. Recent studies examining cross-cultural differences in creativity indicate that societal factors have a significant impact on the formation of new ideas3,4. Creative endeavors, among them dance, music, brainstorming in industries, and scientific collaborations, take place primarily in social settings5. Even when individuals engage in creative activities on their own, their work is often overtly evaluated by peers6 or covertly assessed by internalized cultural norms7. Yet despite the significant role played by social factors in regulating creativity, most research in this field has focused predominantly on isolated individuals, without considering potential group dynamics8. While emerging studies have focused on creativity in dyads9–15,the question of what neural mechanisms form the basis of group creativity remains unresolved.
Given the influence of “esprit de corps” (group mind)16 on individuals within groups, here we adopt an approach that prioritizes the group as the primary unit of analysis. Accordingly, we propose two types of group dynamics that contribute to creativity during group interactions: (1) individuals’ inherent tendency to ‘herd’ and act as a coherent social unit, and (2) individuals’ inclination to act independently17. Herding involves alignment at various levels and includes movement, emotional, and cognitive aspects18. While herding can influence group creativity by enhancing group cooperation, it may also lead individuals to imitate others rather than be original19. Therefore, a mindset of herding would be characterized by high number of similar or imitative responses among group members and lower creative outcomes. On the other hand, independent thinking requires flexibility, exploration of alternative ideas, and the ability to consider diverse possibilities20. Flexibility, which is characterized by being open to different ideas and willing to explore novel approaches, is a fundamental capability that underlies creativity21–23. Studies on group creativity show that that generating creative ideas in a group context requires that members openly share their ideas24–26.
Based on the approach outlined above, we hypothesized the existence of two distinct neural networks—one supporting a herding mindset and the other supporting a flexible group mindset. We further hypothesized that these networks make differential contributions to group creativity (Hypothesis 1). A flexible group mindset enables group members to be receptive to diverse ideas and express their individual perspectives, whereas a herding mindset enables them to adjust their beliefs, attitudes, and behaviors to align with those of others. Consequently, we propose that a higher level of group creativity is associated with a greater degree of flexibility relative to herding (Hypothesis 2). That is, we hypothesize that the relation between the group mindsets of flexibility and herding promotes group creativity.
A key network that may support a flexible mindset during group creativity is the executive control network, which is involved in set shifting, decision-making and working memory27. Neuroimaging studies on creativity have repeatedly found that while Default Mode Network (DMN) that includes regions such as the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), and temporo-parietal junction (TPJ), mediate the generation phase of creative thinking28–32, the Executive Control Network (ECN) is thought to play a critical role in the cognitive processes of evaluating, shifting and selecting ideas4,33. The dorsolateral prefrontal cortex (DLPFC), a core region in the ECN, was found to be activated in studies on creative story generation34, and divergent thinking35. While it was suggested that inhibiting the DLPFC may improve creativity36, different studies have suggested that increased activity in this region is related improvisational music playing37 and insightful problem-solving38,39. Recent research utilizing functional near-infrared spectroscopy (fNIRS) in dyads engaged in dyadic creativity tasks revealed increased interbrain coupling, specifically in the right DLPFC40. Additionally, in comparing group creativity among individuals with different levels of creativity, Xue et al., found that dyads composed of two individuals who were less creative exhibited better cooperation and showed more interbrain coupling in both the right DLPFC and the right TPJ than did dyads containing two highly creative individuals41.Coupled neural activity in time series showing DLPFC brain signals among interacting individuals may represent a coordinated mindset of flexibility and openness. In contrast, interbrain coupling in the observation-execution network may indicate that the group has a herding mindset. Indeed, a recent study showed that interbrain coupling in the inferior frontal gyrus (IFG) is negatively correlated with originality14. It was suggested that the IFG possibly assesses ideas within the context of social norms4. Considering that the IFG is the core region within the mirror neuron system42,43 and that interbrain coupling in this region was found during tasks requiring synchronized behavior44,45, it is possible that interbrain coupling in the IFG is essential for coordination among group members during the creative process. Yet because this region is responsible for imitation and alignment46, inter-brain coupling should be mitigated to achieve original and creative outcomes. Previous research found that lesions in the IFG are associated with increased creativity47 and that cathodal (inhibitory) direct current stimulation (tDCS) targeting this region enhanced creativity30,48. Moreover, while a recent study did not find that cathodal tDCS of the prefrontal cortex increased the novelty of participants’ responses36, a previous study demonstrated that cathodal tDCS over the left IFG enhanced fluency in a divergent-thinking task that required generating uncommon ideas49. On the other hand, it has been demonstrated that anodal tDCS over the left DLPFC facilitates performance in convergent thinking tasks that require creative problem-solving50,51. Hence it is possible that interbrain coupling in the IFG signals a herding mindset in groups and may diminish levels of group creativity.
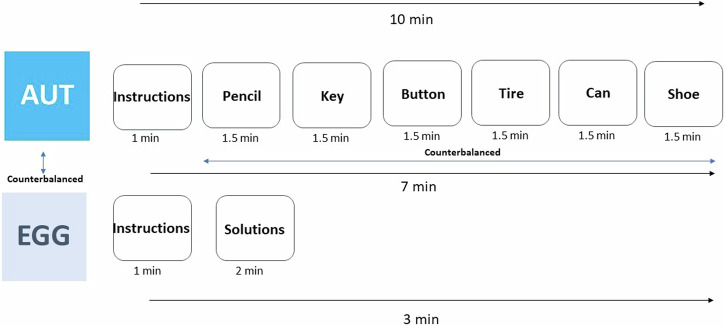
Building on studies reporting that groups of four mammals exhibit complex movement patterns that reflect social interaction dynamics of groups52, the present study aimed to investigate the neural mechanisms underlying creativity within groups of four participants. To evaluate group creativity, we employed two divergent thinking tasks that allow assessing group creativity during discussions (see Methods). We randomly divided 88 participants into groups of four. Each group was tasked with holding discussions to generate innovative ideas and solutions for predefined challenges (Fig. 1). The first task involved brainstorming alternative uses for a common object (e.g., a can), while the second task required devising original strategies to prevent an egg from breaking when dropped from a building. Prior to the discussion phase, we assessed each participant’s individual creativity using established metrics (Torrance) to create a baseline for subsequent comparative analysis. We focused on O2Hb (oxygenated hemoglobin) and posited that engaging in creativity-focused discussions would elicit interbrain coupling in neural structures associated with both herding and flexibility processing. Furthermore, we anticipated that coupling in the DLPFC would be higher than coupling in the IFG when creative output is greater. We predicted that interbrain coupling in the IFG will be associated with a herding mindset characterized by high imitation between group members. Moreover, we predicted that interbrain coupling in the DLPFC would be positively correlated with group creativity, whereas interbrain coupling in the IFG would be negatively correlated with group creativity. Additionally, we predicted that the ratio between the DLPFC and the IFG would predict levels of group creativity.
Fig. 1. Experimental design.

Participants were scanned with functional near-infrared spectroscopy (fNIRS) during a group divergent thinking task. The paradigm involved collaborative discussions in which groups of four participants collectively generated original uses for common objects and collaboratively devised solutions to complex challenges. Individuals signed an informed consent for publication of this image. N = 22 groups.
Consistent with our hypotheses, we observed increased interbrain coupling in both the DLPFC and IFG during brainstorming sessions. Specifically, interbrain coupling in the left DLPFC was significantly higher compared to the left IFG and right IFG. Additionally, our analysis of factors predicting elevated group creativity levels revealed a positive correlation between left DLPFC interbrain coupling and group creativity outcomes, whereas right IFG interbrain coupling was negatively correlated with creativity. These findings align with the concept that low interbrain coupling in the IFG and high interbrain coupling in the DLPFC underlie the dual demands of creative cognition in groups: the propensity to herd with the group and the tendency to generate novel ideas.
Results
Analysis of interbrain coupling
To assess interbrain coupling between individuals in each group, we applied Wavelet Transform Coherence (WTC) to the two neural signal time-series generated from the two regions, implemented using the wavelet coherence package in MATLAB53. These values ranged from 0 (no coherence) to 1 (complete coherence). For each ROI in a group of four participants (A, B, C, D), there were six pairs (AB, AC, AD, BC, BD, CD). We computed the WTC for each pair. To derive a single measure of interbrain coupling for the group for each ROI, we then averaged the WTC values across all pairs. To test whether interbrain coupling in the sample was greater than chance, we created 22 pseudo-groups by randomly pairing fNIRS recordings of four participants, each from a different group. We calculated interbrain coupling of each pseudo-group in a manner that resembled the analysis of the real group.
ANOVA comparison of interbrain coupling between the real and pseudo groups revealed a significant effect for group (F (1,42) = 23.22, p < 0.001, partial η2 = 0.36). The interbrain coupling in the real groups (M = 0.32, SD = 0.01) was significantly higher than in the pseudo groups (M = 0.30, SD = 0.01). There was also a significant effect for brain area (F (1,42) = 16.55, p < 0.001, partial η2 = .28). Interbrain coupling in the DLPFC (M = 0.32, SD = 0.02) was significantly higher than interbrain coupling in the IFG (M = 0.31, SD = 0.01). Lastly, the analysis revealed a significant three-way interaction: group type (real, pseudo) by brain area (IFG, DLPFC) by hemisphere (left, right) interaction (F (1,42) = 4.27, p < 0.05, partial η2 = 0.09).
To examine the source of the interaction, we first explored between-group differences in the various brain regions using one-way multivariate analysis of variance (MANOVA), with group type as the between-subjects independent variable and interbrain coupling in R.IFG, L.IFG, R.DLPFC, and L.DLPFC as the dependent variables. The results revealed a significant difference between real groups and pseudo groups in the R.IFG (F (1,42) = 7.65, p < 0.01, partial η2 = 0.15), L.IFG (F (1,42) = 15.90, p < 0.001, partial η2 = 0.24), R.DLPFC (F (1,42) = 6.95, p < 0.05, partial η2 = 0.14), and L.DLPFC (F (1,42) = 18.20, p < 0.001, partial η2 = 0.30). Interbrain coupling for the real groups was significantly higher than interbrain coupling for the pseudo groups in all regions (see Fig. 2).
Fig. 2. Group differences in interbrain coupling in the different regions.
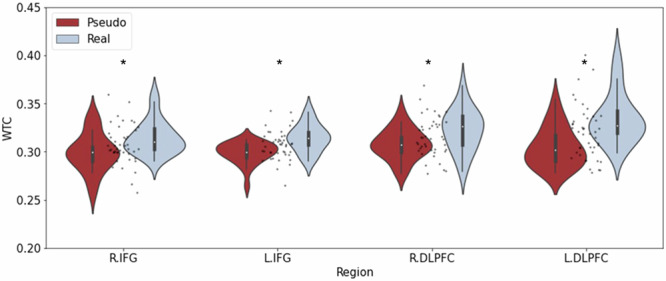
Interbrain coupling for the real groups was significantly higher than interbrain coupling for the pseudo groups in all regions. Asterisks indicate significant difference. N = 22 groups.
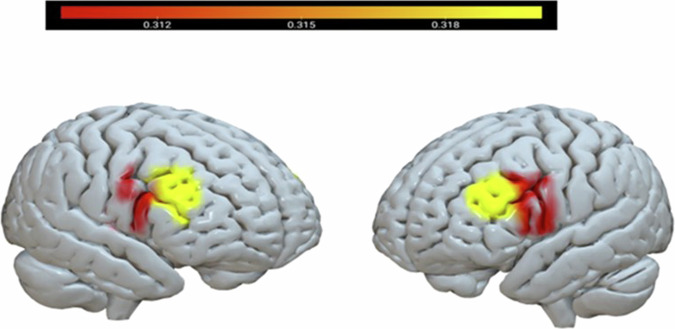
Next, we explored differences between the brain regions separately for each group, using two repeated-measures ANOVAs, with the four brain regions as the between-subjects independent variable and interbrain coupling as the dependent variable. The results revealed a significant effect only for the real groups (F (3,19) = 5.77, p < 0.01, partial η2 = 0.48) but not for pseudo groups (F (3,19) = 2.86, p = 0.064, partial η2 = .31). Post hoc comparisons with Bonferroni correction showed a significant difference between the L.DLPFC and L.IFG (p < 0.05), and between the L.DLPFC and R.IFG (p < 0.01) only for real groups. Interbrain coupling in the L.DLPFC was significantly higher than interbrain coupling in the L.IFG and R.IFG (see Fig. 3).
Fig. 3. Interbrain coupling in the right and left IFG and DLPFC within real groups.
The color intensity is proportional to the interbrain coupling. Red represents lower interbrain coupling. The legend color displays the interbrain coupling value and the corresponding colors. Interbrain coupling in the L.DLPFC was significantly higher than interbrain coupling in the L.IFG and R.IFG. N = 22 groups.
Brain and behavior correlation
We thought to initially examine whether a herding mindset is predicted by interbrain coupling in the IFG. We calculated the number of imitative responses and used stepwise linear regression to identify the specific brain regions that best explain the variance in herding, with herding as the dependent variable and interbrain coupling in the brain regions as the independent variables. The analysis involved iteratively adding variables to the regression equation based on their statistical significance and contribution to the model’s goodness of fit. The results revealed that only the R.IFG (β = 0.55, t = 2.93, p < 0.01) significantly contributed to the model’s goodness of fit. The final regression model including R.IFG was significant (F(1, 20) = 8.61, p < 0.01), with adjusted R2 = 0.27. These results suggest that higher levels of interbrain coupling in R.IFG (positive correlation) predict higher levels of herding (see Fig. 4).
Fig. 4. Herding mindset is predicted by interbrain coupling in the R.IFG.
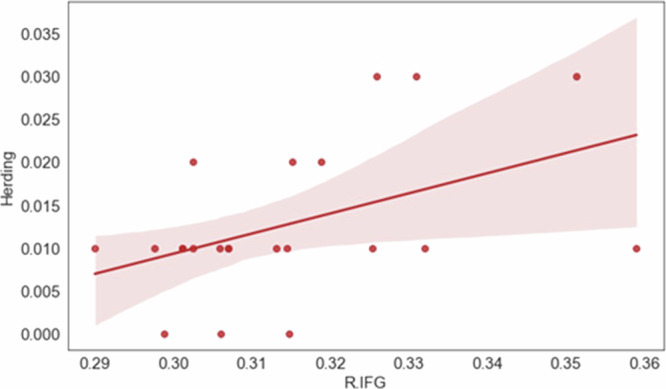
Higher levels of interbrain coupling in R.IFG predict higher levels of herding. N = 22 groups.
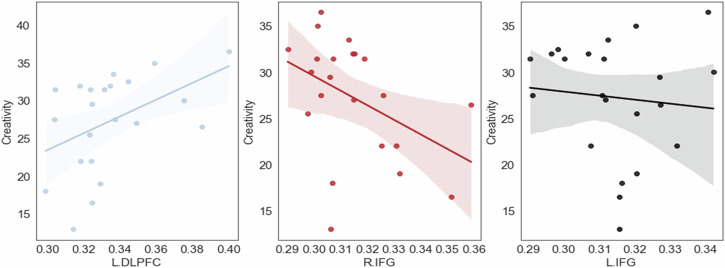
To further identify the set of the brain regions that best explain the variance in creativity, we employed stepwise linear regression, with creativity as the dependent variable and interbrain coupling in the brain regions as the independent variables. The analysis involved iteratively adding variables to the regression equation based on their statistical significance and contribution to the model’s goodness of fit. The results revealed that L.DLPFC (β = 0.73, t = 3.84, p < 0.01), R.IFG (β = −0.42, t = −2.64, p < 0.05), and L.IFG (β = −0.43, t = −2.24, p < 0.05) significantly contributed to the model’s goodness of fit. The final regression model including L.DLPFC, R.IFG, and L.IFG was significant (F(3, 18) = 7.34, p < 0.01), with adjusted R2 = 0.48. These results suggest that higher levels of interbrain coupling in L.DLPFC (positive correlation) and lower levels of interbrain coupling in R.IFG and L.IFG (negative correlation) predict higher levels of creativity (see Fig. 5).
Fig. 5. The brain regions that predict the variance in creativity.
Higher levels of interbrain coupling in L.DLPFC and lower levels of interbrain coupling in R.IFG predict higher levels of creativity. N = 22 groups.
Next, we examined our hypothesis that higher creativity / lower herding within a group would be associated with higher / lower interbrain coupling of the R/L.DLPFC relative to the interbrain coupling of the R.IFG and L.IFG.
To identify which specific ratio explains the variance in herding, we employed stepwise linear regression, with herding as the dependent variable and the ratios as the independent variables. The analysis involved iteratively adding variables to the regression equation based on their statistical significance and contribution to the model’s goodness of fit. The results revealed that only the L.DLPFC/R.IFG ratio (β = −0.50, t = −2.59, p < 0.05) significantly contributed to the model’s goodness of fit. The final regression model including the L.DLPFC/R.IFG ratio was significant (F(1, 20) = 6.73, p < 0.05), with adjusted R2 = 0.21. The results suggest that higher levels of balance between L.DLPFC and R.IFG predict lower levels of herding (see Fig. 6).
Fig. 6. The variance in herding is explained by L.DLPFC/R.IFG ratio.
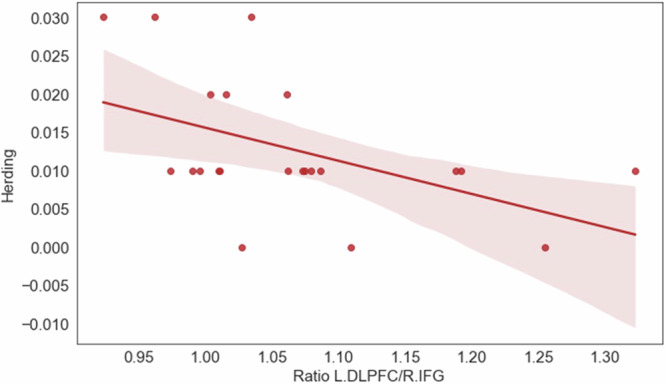
Higher levels of balance between L.DLPFC and R.IFG predict lower levels of herding. N = 22 groups.
To identify which ratio explains the variance in creativity, we employed stepwise linear regression, with creativity as the dependent variable and the ratios as the independent variables. The analysis involved iteratively adding variables to the regression equation based on their statistical significance and contribution to the model’s goodness of fit. The results revealed that only the L.DLPFC/R.IFG ratio (β = 0.63, t = 3.65, p < 0.01) significantly contributed to the model’s goodness of fit. The final regression model including the L.DLPFC/R.IFG ratio was significant (F(1, 20) = 13.32, p < 0.01), with adjusted R2 = 0.37. The results suggest that higher levels of balance between L.DLPFC and R.IFG predict higher levels of creativity (see Fig. 7).
Fig. 7. The variance in creativity is explained by L.DLPFC/R.IFG ratio.
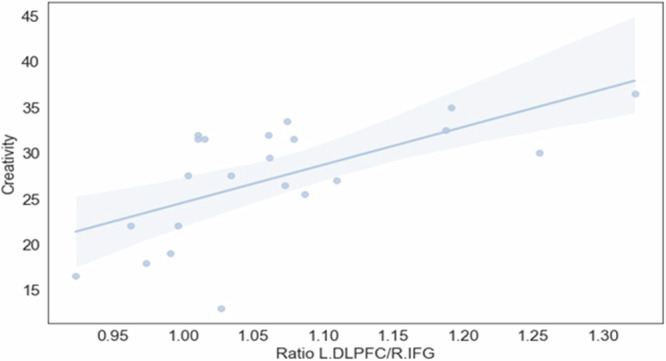
Higher levels of balance between L.DLPFC and R.IFG predict higher levels of creativity. N = 22 groups.
Next, we examined whether the mean individual creativity in each group explains the variance of group creativity using Linear regression model. The regression model, with group creativity as the dependent variable and the mean individual creativity in each group as the independent variable revealed no significant effect on group creativity (F(1, 20) = 0.15, p = 0.704).
Lastly, we examined whether interbrain coupling explain the variance of individual creativity using Linear mixed (LME) model. The LME model, with individual creativity as the dependent variable, interbrain coupling in the brain regions as the independent variables, and group ID as random effect, also showed no significant effect of L.DLPFC (F(1, 17) = 0.33, p = 0.576), R.DLPFC (F(1, 17) = 0.00, p = 0.978), R.IFG (F(1, 17) = 0.34, p = 0.568), and L.IFG (F(1, 17) = 0.13, p = 0.725) on individual creativity.
Discussion
In the current study we aimed to examine interbrain coupling in areas associated with a group mindset of flexibility and herding and to investigate how interbrain coupling contributes to group creativity. The neuroimaging findings revealed an increase in inter-brain coupling among pairs across regions. This phenomenon was identified by contrasting the interbrain coupling in real vs. pseudo dyads where interaction was lacking. This approach allowed ruling out the possibility interbrain coupling merely reflects general brain activity stemming from task engagement, irrespective of actual interactive participation. Interbrain coupling in the left and right IFG in the creativity condition for the real groups compared to the pseudo groups is particularly interesting in light of the IFG’s established association with synchronization54,55 and cooperation14. Neuroimaging studies of imitation56 and emotional empathy57 have consistently exhibited activation in the IFG. Although studies of synchronization have largely focused on physical movement synchronization45,58, Shamay-Tsoory et al.18 suggested that emotion-based synchronization (e.g., emotional contagion) may be conceptually similar to physical synchronization. The recruitment of the IFG during group discussions gives some credence to the notion that interbrain coupling in the IFG supports different types of synchronization.
If interbrain coupling in the IFG reflects the group members’ herding mindset during group discussions, we might expect interbrain coupling in this region to correlate negatively with the subsequent creative outputs. Indeed, at the group level, those groups with stronger interbrain coupling in the IFG had lower creativity scores. Our findings are in line with a previous study conducted by Mayseless et al.14, which also reported a negative correlation between interbrain coupling in the IFG and creativity scores. Similarly, Gelfand et al.59 found that groups instructed to engage in synchronized walking demonstrated lower levels of creativity than did unsynchronized groups. Therefore, our results support the notion that synchronized behavior may have a detrimental effect on group creativity. Notably, the involvement of the right IFG is consistent with its recognized roles in imitation, coordination, intention coding, and perception-action matching60. Distinctly, interbrain coupling in the left IFG has been associated with verbal synchronization during verbal tasks61. whereas the right IFG has been linked to non-verbal coordination62. This suggests that elevated interbrain coupling in the right IFG may predominantly enhance non-verbal imitation which could detrimentally impact group creativity.
In contrast to the IFG, the DLPFC has been repeatedly linked to cognitive processes such as flexibility and executive control63,64. Thus, the interbrain coupling in the DLPFC observed here during the creativity discussions may be related to a flexible mindset of the group, allowing multiple divergent ideas to emerge. Higher cognitive demands may be placed on participants in order for them to overcome the strong tendency to imitate each other. The positive correlation we found between creativity and interbrain coupling in the left DLPFC supports this interpretation, suggesting that interbrain coupling in the left DLPFC is associated with facilitating creative thinking by enabling cognitive flexibility and overcoming imitative tendencies. The lack of correlation between creativity and interbrain coupling in the right DLPFC may be attributed to the specific nature of the creativity task used in this study. The study employed the AUT, which is a verbal divergent thinking task. A meta-analysis showed that the left DLPFC is associated with verbal divergent thinking, while the right DLPFC is linked to visual divergent thinking65.
Interestingly, our finding suggest that interbrain coupling is not linked to individual creativity. This suggest that during group interaction the group mindset contributes more to collective outcomes than individual creativity. In exploring the neural correlates of creativity, both individual and group dynamics offer distinct insights. The DMN, known to mediate associative thinking33 is crucial for generating potential ideas from long-term memory. Concurrently, the ECN facilitates cognitive processes involving the transition between and selection of these creative thoughts4. During individual creativity, the DLPFC is particularly critical, promoting cognitive flexibility necessary for selection and transition between ideas. In group settings, DLPFC activity, when coupled across individuals, facilitates a synchronized, flexible mindset through interbrain coupling, enhancing collective creativity. Meanwhile, the Observation-Execution system, prominently involving the IFG, is crucial for evaluating ideas and aligning ideas with internalized norms during individual creativity and externally presented group norms during group creativity. However, while interbrain coupling in the IFG supports social coherence, it may also suppress novelty, leading to mimicry rather than innovation. Thus, we hold that optimal group creativity is achieved by balancing interbrain connectivity: enhanced DLPFC coupling fosters flexible idea navigation, whereas diminished IFG interbrain coupling reduces conformity, promoting originality and diversity in creative outputs.
Motivated by the hypothesis that interbrain coupling in the DLPFC may be competing with the IFG in creativity, we examined the relationship between interbrain coupling in the IFG and the DLPFC during creativity discussions by measuring the ratio between the left DLPFC and the right IFG. We found that the ratio between interbrain coupling in the right IFG and the left DLPFC was the most significant predictor of creativity performance. This suggests that the creative outcomes of groups may reflect the relative coupling of these regions, with greater DLPFC coupling biased toward high creativity and greater interbrain coupling in the IFG biased toward low creativity. These findings are highly relevant to understanding how group dynamics affect creativity during group interactions. A related study by Choi et al.66 reported that groups marked by collective value orientation and independent self-representation generate more original ideas than groups that are manipulated to be collective but interdependent. Therefore, creating an environment that encourages both collective value orientation and independent thinking with greater emphasis on independent thinking may be a promising strategy to boost group creativity, as suggested by our results showing a balance between interbrain coupling in the IFG and the DLPFC.
There are several limitations that need to be acknowledged in this study. First, the majority of participants in our sample were female. Previous research has pointed to potential gender differences in creativity performance67 as well as in neuroimaging investigations of creative thinking68. It is important for future research to explore our brain model of group creativity across diverse samples, including a wider range of gender representation, in order to validate and extend the findings to different populations. Finally, note that the duration of the task in our study was relatively short, lasting eight minutes. Exploring the impact of different task durations on group dynamics and creativity could provide valuable insights into the temporal aspects of group creative processes.
Our results are in line with the notion that the IFG and DLPFC represent the twin demands of creative cognition in groups: the tendency to herd with the group and the cognitive goal of generating novel ideas. We believe that these findings, and those of related future studies, will provide a more detailed characterization of specific social elements, their neural substrates, and the social circumstances that affect group creativity. Therefore, not only do our results provide direct empirical support for models of creativity that acknowledge the influence of social interactions, they also serve as a first step toward understanding the neural underpinnings of group dynamics.
Materials and methods
Participants
The sample consisted of 88 participants (12 males, 76 females; average age: 22.97 + 4.28). All participants were right-handed, had normal or corrected-to-normal vision, and reported no history of neurological or psychiatric conditions. They were recruited through advertisements posted at the University of Haifa and assigned into groups based on gender, with three male groups and 19 female groups, each containing four participants. In each group, the participants were not familiar with each other. Participants were compensated for their participation either with monetary payment or with academic credit points, if applicable. The study was approved by the local Ethics Committee of the University of Haifa, and participants joined the study only after signing an informed consent form. All ethical regulations relevant to human research participants were followed.
Measures
Measurement of creativity
Group creativity
Two divergent creative tasks were administered to assess group creativity.
The Alternate Uses Task (AUT69): The experimenter named six common objects (pencil, key, button, tire, can, and shoe) to the groups, one at a time, together with the most common use for each object. They were also given an example of an alternate use for an object (making holes in a bottle of water and transforming it into a sprinkler). The groups were then given 90 seconds70 to collectively generate alternative uses for each object. The participants were explicitly instructed to suggest alternative uses that had not been previously considered. To prevent an order effect (response change due to fatigue, practice, carry over effect or other factors71) two versions of this task were created entailing the same six objects presented in different order. Each version was administered to half of the groups.
Egg task72: The groups were given two minutes to collectively think of original ways to prevent an egg from breaking after falling from a ten-meter-high building. To make sure the participants understood the task, they were given the example of taming an eagle to catch the egg when it falls.
In both creativity tasks, the participants were informed that in the realm of appropriate and possible suggestions there were no correct or incorrect answers, and they were encouraged to generate as many responses as possible. Additionally, to ensure the active participation of all participants in the tasks, each round began with each individual offering a response. After this initial contribution, the brainstorming session was open for free collaboration among the group members. Participants were explicitly instructed to disregard the presence of the experimenter and to engage in group brainstorming while generating alternative uses (see Fig. 8).
Fig. 8. Creativity tasks.
The creativity tasks were counterbalanced between groups. In the AUT, objects were counterbalanced between groups. N = 22 groups.
Scoring: The verbal responses of participants within each group were captured via audio recording and subsequently transcribed verbatim to facilitate the coding process. The transcription was undertaken to precisely attribute each response to its respective participant within the group dynamics.
Creativity scores were assessed by expert raters according to two dimensions: flexibility (number of categories) and originality (unique responses). Originality was scored by considering statistically infrequent responses. The raters underwent training that involved familiarization with the assessment criteria and calibration exercises to align their ratings. Frequency analysis was conducted for each response across all participants. Responses given by more than 10% of the participants were assigned 0 points, responses given by 5% to 10% were assigned 1 point, and responses provided by less than 5% were assigned 2 points. The originality score was then calculated as the cumulative number of points across all responses. The originality and flexibility scores were calculated for each task. The total creativity score for each task was computed as the mean of the originality and flexibility scores. The final creativity score used for each group in the analysis was the sum of the total creativity scores on both tasks. This decision was influenced by our prior identification of a significant correlation between these tasks, both of which involve divergent thinking73.
Table 1 shows the means (and standard deviations) of the originality, flexibility, and total creativity scores of the AUT and EGG tasks for the groups.
Table 1.
Means (and standard deviations) of the creativity measures
| Variables | AUT | EGG | Total |
|---|---|---|---|
| Originality | 15.91 (7.41) | 0.45 (0.8) | 16.36 (7.74) |
| Flexibility | 34.23 (7.62) | 3.95 (1.17) | 38.18 (7.76) |
| Total creativity | 25.07 (6.17) | 2.20 (0.73) | 27.27 (6.38) |
Note. The table displays means and standard deviations (in parentheses) for the creative tasks and a total creative score of the groups. N = 22 groups.
Individual creativity
To assess Individual creativity, we used a subset of the Torrance Test of Creative Thinking (TTCT)74 in which individuals are instructed to draw as many objects or pictures as possible, using thirty-six circles as the main part of their drawings. Participants were asked to write down a name for each drawing next to it. They were given ten minutes to complete the task. Responses were rated by an expert rater for flexibility and originality according to the scoring guide74. Flexibility represents the number of different categories into which the participant’s responses can be classified. For example, glasses, clown, and sunflower were divided into three different categories (apparel, human beings and flower), while rose, sunflower and flower were assigned to the same category (flower). Sixty-eight categories were derived from the manual, and we added two additional categories (fictional characters; computers and smartphones), for a total of seventy categories. As indicated by the manual, originality is measured by ideas that are statistically infrequent: Responses given by 10% or more of the participants are awarded no credit, responses provided by 5–9.99% are awarded one point, responses provided by 2-4.99% are awarded two points, and responses provided by less than 2% are awarded three points. The originality score is the total of the points summed across all responses. The total creativity score was calculated as the mean of the final originality and flexibility scores.
Assessment of herding
To evaluate the levels of group herding we developed a measurement that represents the number of responses that were repeated during group discussions for each AUT item.
The herding score was quantified by a trained rater who calculated the recurrence of identical or conceptually similar responses among participants within the same group. Responses that were verbatim repetitions, such as multiple participants independently suggesting an identical item (e.g., “a chair”), were allocated a score of 1 point. Conversely, responses that shared a thematic similarity but varied in expression, such as one participant proposing “a chair” and another describing the use of the chair for sitting and skating, were assigned a score of 0.5 points. The aggregate herding score for the group was computed as the mean point value across all evaluated responses.
Procedure
Upon arriving at the laboratory, each of the four group participants signed consent forms and completed demographic questions. Next, each participant received instructions for the TTCT task. The participants were then fitted with fNIRS caps and were instructed not to interact with each other unless explicitly instructed otherwise. They were then informed that they would be engaging in two joint divergent creativity tasks and were seated face-to-face around a table. To mitigate any potential effects of one task on the other, the order of the two creativity tasks was counterbalanced. Instructions for both tasks were delivered verbally, and participants’ behavioral and neuronal responses were recorded.
Calculation of group interbrain coupling
For each participant, we measured the O2Hb (oxygenated hemoglobin) time series of the four regions. We used a Morlet wavelet function in the frequency range of 0.015 to 0.15 Hz as the mother wavelet. This range was because it falls outside the typical frequencies associated with heart rate (around 1–2 Hz) and breathing (around 0.2–0.3 Hz), which are common sources of noise in fNIRS data75.For each dyad of participants in each group, we calculated the interbrain coupling measure for each combination of homologous regions. We then averaged group interbrain coupling values during both creativity tasks (AUT, EGG task) to extract a group-level interbrain coupling value.
fNIRS data acquisition
We measured interbrain coupling between the brains of the four members of each group (group) using the Brite24 Functional near-infrared spectroscopy (fNIRS) system (Artinis Medical Systems; Elst, The Netherlands). This system is used to simultaneously measure changes in oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb) concentrations in the cortex. Both O2Hb and HHb signals can be employed to measure changes in cerebral blood flow. Here we focused mainly on the O2Hb signal, since research has shown it to be more sensitive to changes in cerebral blood flow12,61,76. The system employs near-infrared light transmission at two wavelengths, 760 and 850 nm.
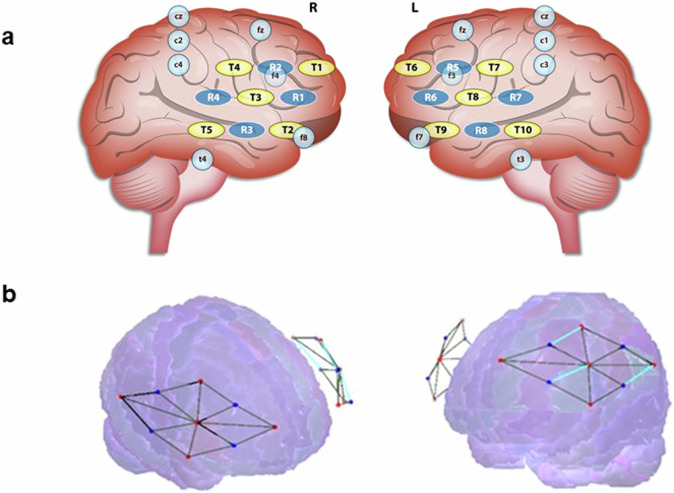
Measurements were taken at a 50 Hz rate. The system consists of a flexible probe unit (cap) with 18 optodes:10 transmitters and 8 receivers. Each pairing of a transmitter and receiver forms a channel, resulting in 12 channels in each hemisphere. Optode location was chosen using the international EEG 10–20 system, partially covering the bilateral prefrontal cortex (see Fig. 9a). Channels were later clustered into regions of interest (ROIs) of each transmitter with all its receivers, based on the estimated Broadmann’s area over which they were placed. The bilateral ROIs included the Premotor Cortex (PMC), the opercular part of Inferior Frontal Gyrus (IFG’s pars opercularis; BA44), the triangular part of the IFG (pars triangularis; BA45), the Dorsolateral Prefrontal Cortex’s (DLPFC) Broadmann’s area 46, and the DLPFC’s Broadmann’s area 9 (see Fig. 9b).
Fig. 9. fNIRS Channels configuration.
a Optode montage relative to the EEG 10/20 system. T represent a transmitter and R represent a receiver. T 2 and 3 were considered the left IFG; T 9 and 8 were considered the right IFG. T 6 was considered the left DLPFC; T 1 was considered the right DLPFC. b Illustration of the Brite24 fNIRS system channels montage. Red represents sources and blue represents detectors; together they form 24 channels. Black lines are the channels, optode projections from scalp to cortex. N = 22 groups.
Neural data preprocessing
A visual scan was conducted using HOMER 377. In addition, we implemented the following five-step data preprocessing pipeline: 1) Converting the light intensity data to optical density, needed later to obtain accurate measurements of oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb) concentrations. 2) Identifying motion artifacts and correcting them using a targeted principal component analysis (PCA) approach78. 3) Applying a bandpass filter to the data (cutoff frequencies of 0 to 1 Hz) to help remove high-frequency components in the signal that are unrelated to brain activity79. 4) Converting the optical density data to O2Hb and HHb concentration values that capture cerebral blood flow (a proxy for neural activity) using the modified Beer–Lambert law80. 5) Removing channels that exhibit a positive correlation above 0.5 between O2Hb and HHb, based on the assumption that oxy (O2Hb) and deoxy (HHb) hemoglobin typically exhibit a strong negative correlation81 (58). This preprocessing pipeline ensures higher quality data that is suitable for further analysis.
Statistical analysis
To examine differences between real and pseudo groups in average interbrain coupling during the creative tasks, we initially employed a mixed-design repeated-measures ANOVA, with group (real, pseudo) as the between-subjects factor and brain area (IFG, DLPFC) and hemisphere (left, right) as the within-subject factors. We then calculated Pearson correlations between the groups’ interbrain coupling and creativity results. To predict herding and creativity performance, we conducted two stepwise linear regression, with herding and creativity scores as the dependent variable and interbrain coupling in all the regions as predictors. Additionally, we calculated the balance between R/L.DLPFC and R.IFG and the balance between R/L.DLPFC and L.IFG by dividing the interbrain coupling of R/L.DLPFC by the interbrain coupling of R.IFG and dividing the interbrain coupling of R/L.DLPFC by the interbrain coupling of L.IFG. Using these four ratio measures, we used two stepwise linear regression predict creativity and herding results. Finally, one LME model was used to predict individual creativity based on interbrain coupling, with group ID as random effect, and one linear regression model was used to predict group creativity based on the mean individual creativity in each group.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Materials
Acknowledgements
This study was funded by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (grant agreement no. INTERPLASTIC: 101020091; DLV-101020091) and a grant from the Israel Science Foundation (ISF) grant 959/18.
Author contributions
Conceptualization: S.S.T., N.F., H.P. Methodology: S.S.T., N.F., H.P. Investigation: D.Z., N.F., H.P. Visualization: N.F., H.P. Supervision: S.S.T. Writing—original draft: S.S.T., N.F., H.P. Writing—review & editing: S.S.T., N.F., H.P.
Peer review
Peer review information
Communications Biology thanks Marcela Ovando-Tellez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Michel Thiebaut de Schotten and Benjamin Bessieres. A peer review file is available.
Data availability
The datasets analyzed during this study is available at 10.5281/zenodo.12200031 (https://zenodo.org/records/12200032). The source data behind the graphs and table in the paper is available in Supplementary Data 1. Other data will be available from the corresponding author on reasonable request.
Code availability
The codes generated during this study will be available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hadas Pick, Nardine Fahoum.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-06614-7.
References
- 1.Gabora, L. Creativity Cultural Evolution Gabora Creativity: Linchpin in the Quest for a Viable Theory of Cultural Evolution. Current Opinion in Behavioral Sciences vol. 27.
- 2.Sawyer, R. K. Creativity Research and Cultural Context: Past, Present, and Future. In Journal of Creative Behavior vol. 51 352–354 (Creative Education Foudation, 2017).
- 3.Ivancovsky, T., Shamay-Tsoory, S., Lee, J., Morio, H. & Kurman, J. A dual process model of generation and evaluation: A theoretical framework to examine cross-cultural differences in the creative process. Pers. Individ Dif.139, 60–68 (2019). 10.1016/j.paid.2018.11.012 [DOI] [Google Scholar]
- 4.Kleinmintz, O. M., Ivancovsky, T. & Shamay-Tsoory, S. G. The twofold model of creativity: the neural underpinnings of the generation and evaluation of creative ideas. Curr. Opin. Behav. Sci.27, 131–138 (2019). 10.1016/j.cobeha.2018.11.004 [DOI] [Google Scholar]
- 5.Engeström, Y. Expansive Learning at Work: Toward an activity theoretical reconceptualization. J. Educ. Work14, 133–156 (2001). 10.1080/13639080020028747 [DOI] [Google Scholar]
- 6.Csikszentmihalyi, M. The flow experience and its significance for human psychology. Optim. Experience: Psychological Stud. Flow. Conscious.2, 15–35 (1988). 10.1017/CBO9780511621956.002 [DOI] [Google Scholar]
- 7.Ivancovsky, T., Shamay‐Tsoory, S., Lee, J., Morio, H. & Kurman, J. A Multifaceted Approach to Measure Creativity across Cultures: The Role of the Centrality of Context in Divergent Thinking Tasks. J. Creat Behav. jocb.506 10.1002/jocb.506. (2021).
- 8.Beaty, R. E., Benedek, M., Silvia, P. J. & Schacter, D. L. Creative Cognition and Brain Network Dynamics. Trends Cogn. Sci.20, 87–95 (2016). 10.1016/j.tics.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balters, S., Hawthorne, G. & Reiss, A. L. Priming Activity to Increase Interpersonal Closeness, Inter-Brain Coherence, and Team Creativity Outcome. In 227–241. 10.1007/978-3-031-36103-6_12 (2023).
- 10.Duan, H. et al. Is the creativity of lovers better? A behavioral and functional near-infrared spectroscopy hyperscanning study. Curr. Psychol.41, 41–54 (2022). 10.1007/s12144-020-01093-5 [DOI] [Google Scholar]
- 11.Cheng, X., Li, X. & Hu, Y. Synchronous Brain Activity during Cooperative Exchange Depends on Gender of Partner: A fNIRS-based Hyperscanning Study. 10.1002/hbm.22754. [DOI] [PMC free article] [PubMed]
- 12.Czeszumski, A. et al. Cooperative Behavior Evokes Interbrain Synchrony in the Prefrontal and Temporoparietal Cortex: A Systematic Review and Meta-Analysis of fNIRS Hyperscanning Studies. eNeuro9, 0268–21 (2022). [DOI] [PMC free article] [PubMed]
- 13.Lu, K. & Hao, N. When do we fall in neural synchrony with others? Soc. Cogn. Affect Neurosci.14, 253–261 (2019). 10.1093/scan/nsz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayseless, N., Hawthorne, G. & Reiss, A. L. NeuroImage Real-life creative problem solving in teams: fNIRS based hyperscanning study. Neuroimage203, 116161 (2019). 10.1016/j.neuroimage.2019.116161 [DOI] [PubMed] [Google Scholar]
- 15.Yin, J., Pan, Y., Zhang, Y., Hu, Y. & Luo, J. Distinct inter-brain synchronization patterns during group creativity under threats in cooperative and competitive contexts. Think Skills Creat49, 101366 (2023).
- 16.Durkheim, E. Emile Durkheim on Morality and Society. (University of Chicago Press., 1973).
- 17.Mayo, O. & Gordon, I. In and out of synchrony—Behavioral and physiological dynamics of dyadic interpersonal coordination. Psychophysiology57, 1–15 (2020). 10.1111/psyp.13574 [DOI] [PubMed] [Google Scholar]
- 18.Shamay-Tsoory, S. G., Saporta, N., Marton-Alper, I. Z. & Gvirts, H. Z. Herding Brains: A Core Neural Mechanism for Social Alignment. Trends Cogn. Sci.23, 174–186 (2019). 10.1016/j.tics.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 19.Fahoum, N., Pick, H., Ivancovsky, T. & Shamay-Tsoory, S. Free your mind: creative thinking contributes to overcoming conflict-related biases. Brain Sci. 12, 1566. 10.3390/brainsci12111566 (2022). [DOI] [PMC free article] [PubMed]
- 20.Orakcı, Ş. Exploring the relationships between cognitive flexibility, learner autonomy, and reflective thinking. Think Skills Creat41, 100838 (2021).
- 21.Kloo, D., Perner, J., Aichhorn, M. & Schmidhuber, N. Perspective taking and cognitive flexibility in the Dimensional Change Card Sorting (DCCS) task. Cogn. Dev.25, 208–217 (2010). 10.1016/j.cogdev.2010.06.001 [DOI] [Google Scholar]
- 22.Stahl, L. & Pry, R. Attentional flexibility and perseveration: Developmental aspects in young children. Child Neuropsychol.11, 175–189 (2005). 10.1080/092970490911315 [DOI] [PubMed] [Google Scholar]
- 23.Stevens, A. D., Wilson, B. J., Skidmore, J. R., Frey, K. & Roe, M. D. Social Problem-Solving and Cognitive Flexibility: Relations to Social Skills and Problem Behavior of At-Risk Young Children. (2009).
- 24.Camacho, L. M. & Paulus, P. B. The Role of Social Anxiousness in Group Brainstorming. Journal of Personality and Social Psychology vol. 68 (1995).
- 25.Nijstad, B. A. & De Dreu, C. K. Motivated information processing in organizational teams: Progress, puzzles, and prospects. Res. Organ. Behav. 32, 87–111. 10.1016/j.riob.2012.11.004 (2012).
- 26.Reiter-Palmon, R. & Japp, P. Creativity and Innovation in Groups. In Group Communication 219–232 (Routledge, 2023).
- 27.Chrysikou, E. G. The Costs and Benefits of Cognitive Control for Creativity. In The Cambridge Handbook of the Neuroscience of Creativity. (Cambridge University Press, 2018).
- 28.Kühn, S. et al. The Importance of the Default Mode Network in Creativity—A Structural MRI. Study J. Creat. Behav.48, 152–163 (2014). 10.1002/jocb.45 [DOI] [Google Scholar]
- 29.Marron, T. R. et al. Chain free association, creativity, and the default mode network. Neuropsychologia118, 40–58 (2018). 10.1016/j.neuropsychologia.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 30.Mayseless, N., Eran, A. & Shamay-Tsoory, S. G. Generating original ideas: The neural underpinning of originality. Neuroimage116, 232–239 (2015). 10.1016/j.neuroimage.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 31.Raichle, M. E. et al. A default mode of brain function. Proc. Natl. Acad. Sci. Usa.98, 676–682 (2001). 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu, W. et al. Common and distinct brain networks underlying verbal and visual creativity. Hum. Brain Mapp.38, 2094–2111 (2017). 10.1002/hbm.23507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaty, R. E. & Kenett, Y. N. Associative thinking at the core of creativity. Trends Cogn. Sci.27, 671–683 (2023). 10.1016/j.tics.2023.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Howard-Jones, P. A., Blakemore, S. J., Samuel, E. A., Summers, I. R. & Claxton, G. Semantic divergence and creative story generation: An fMRI investigation. Cogn. Brain Res.25, 240–250 (2005). 10.1016/j.cogbrainres.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 35.Carlsson, I., Wendt, P. E. & Risberg, J. On the Neurobiology of Creativity. Di€erences in Frontal Activity between High and Low Creative Subjects. www.elsevier.com/locate/neuropsychologia. [DOI] [PubMed]
- 36.Kenett, Y. N., Rosen, D. S., Tamez, E. R. & Thompson-Schill, S. L. Noninvasive brain stimulation to lateral prefrontal cortex alters the novelty of creative idea generation. 10.3758/s13415-021-00869-x/Published. (2021) [DOI] [PMC free article] [PubMed]
- 37.Bengtsson, S. L., Csíkszentmihályi, M. & Ullén, F. Cortical regions involved in the generation of musical structures during improvisation in pianists. J. Cogn. Neurosci.19, 830–842 (2007). 10.1162/jocn.2007.19.5.830 [DOI] [PubMed] [Google Scholar]
- 38.Kounios, J. et al. The origins of insight in resting-state brain activity. Neuropsychologia46, 281–291 (2008). 10.1016/j.neuropsychologia.2007.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramaniam, K., Kounios, J., Parrish, T. B. & Jung-Beeman, M. A Brain Mechanism for Facilitation of Insight by Positive Affect. http://direct.mit.edu/jocn/article-pdf/21/3/415/1937443/jocn.2009.21057.pdf. [DOI] [PubMed]
- 40.Lu, K., Xue, H., Nozawa, T. & Hao, N. Cooperation Makes a Group be More Creative. Cerebral Cortex 1–14 10.1093/cercor/bhy215 (2018). [DOI] [PubMed]
- 41.Xue, H., Lu, K. & Hao, N. Cooperation makes two less-creative individuals turn into a highly-creative pair. Neuroimage172, 527–537 (2018). 10.1016/j.neuroimage.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 42.Gallese, V. Before and below ‘theory of mind’: Embodied simulation and the neural correlates of social cognition. In Philosophical Transactions of the Royal Society B: Biological Sciences vol. 362 659–669 (Royal Society, 2007). [DOI] [PMC free article] [PubMed]
- 43.Rizzolatti, G. & Craighero, L. The Mirror-Neuron System. Annu. Rev. Neurosci.27, 169–192 (2004). 10.1146/annurev.neuro.27.070203.144230 [DOI] [PubMed] [Google Scholar]
- 44.Osaka, N. et al. How two brains make one synchronized mind in the inferior frontal cortex: FNIRS-based hyperscanning during cooperative singing. Front Psychol.6, 1811 (2015). [DOI] [PMC free article] [PubMed]
- 45.Gamliel, H. N. et al. Inter-group conflict affects inter-brain synchrony during synchronized movements. Neuroimage245, 118661 (2021). [DOI] [PubMed]
- 46.Iacoboni, M. & DaprettoIacoboni, M. The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci.7, 942–951 (2006). 10.1038/nrn2024 [DOI] [PubMed] [Google Scholar]
- 47.Shamay-Tsoory, S. G., Adler, N., Aharon-Peretz, J., Perry, D. & Mayseless, N. The origins of originality: The neural bases of creative thinking and originality. Neuropsychologia49, 178–185 (2011). 10.1016/j.neuropsychologia.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 48.Ivancovsky, T., Kurman, J., Morio, H. & Shamay-Tsoory, S. Transcranial direct current stimulation (tDCS) targeting the left inferior frontal gyrus: Effects on creativity across cultures. Soc. Neurosci.14, 277–285 (2019). 10.1080/17470919.2018.1464505 [DOI] [PubMed] [Google Scholar]
- 49.Chrysikou, E. G. et al. Noninvasive transcranial direct current stimulation over the left prefrontal cortex facilitates cognitive flexibility in tool use. Cogn. Neurosci.4, 81–89 (2013). 10.1080/17588928.2013.768221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metuki, N., Sela, T. & Lavidor, M. Enhancing cognitive control components of insight problems solving by anodal tDCS of the left dorsolateral prefrontal cortex. Brain Stimul.5, 110–115 (2012). 10.1016/j.brs.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 51.Zmigrod, S., Colzato, L. S. & Hommel, B. Stimulating Creativity: Modulation of Convergent and Divergent Thinking by Transcranial Direct Current Stimulation (tDCS). 10.1080/10400419.2015.1087280 (2015).
- 52.Shemesh, Y. et al. High-order social interactions in groups of mice. Elife2, 1–19 (2013). 10.7554/eLife.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grinsted, A., Moore, J. C. & Jevrejeva, S. Nonlinear Processes in Geophysics Application of the Cross Wavelet Transform and Wavelet Coherence to Geophysical Time Series. vol. 11 http://www.pol.ac.uk/home/research/waveletcoherence/ (2004).
- 54.Sasaki, A. T., Okamoto, Y., Kochiyama, T., Kitada, R. & Sadato, N. Distinct sensitivities of the lateral prefrontal cortex and extrastriate body area to contingency between executed and observed actions. Cortex108, 234–251 (2018). 10.1016/j.cortex.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 55.Caspers, S., Zilles, K., Laird, A. R. & Eickhoff, S. B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage50, 1148–1167 (2010). 10.1016/j.neuroimage.2009.12.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iacoboni, M. et al. Cortical mechanisms of human imitation. Science (1979)286, 2526–2528 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Shamay-Tsoory, S. G., Aharon-Peretz, J. & Perry, D. Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain132, 617–627 (2009). 10.1093/brain/awn279 [DOI] [PubMed] [Google Scholar]
- 58.Saporta, N. et al. Altered activation in the action observation system during synchronization in high loneliness individuals. Cereb. Cortex33, 385–402 (2023). 10.1093/cercor/bhac073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelfand, M. J., Caluori, N., Jackson, J. C. & Taylor, M. K. The cultural evolutionary trade-off of ritualistic synchrony: CULTURAL TRADEOFF of SYNCHRONY. Philosophical Transactions Royal Society B: Biol. Sci.375, 20190432 (2020). [DOI] [PMC free article] [PubMed]
- 60.Wei, Y. et al. Reduced interpersonal neural synchronization in right inferior frontal gyrus during social interaction in participants with clinical high risk of psychosis: An fNIRS-based hyperscanning study. Prog. Neuro-Psychopharmacol. Biol. Psych.120, 110634 (2023). 10.1016/j.pnpbp.2022.110634 [DOI] [PubMed] [Google Scholar]
- 61.Jiang, J. et al. Neural Synchronization during Face-to-Face Communication. 32, 16064–16069 (2012). [DOI] [PMC free article] [PubMed]
- 62.Minagawa, Y., Xu, M. & Morimoto, S. Toward Interactive Social Neuroscience: Neuroimaging Real-World Interactions in Various Populations. Jpn. Psychol. Res.60, 196–224 (2018). 10.1111/jpr.12207 [DOI] [Google Scholar]
- 63.Gerlach, K. D., Spreng, R. N., Gilmore, A. W. & Schacter, D. L. Solving future problems: Default network and executive activity associated with goal-directed mental simulations. Neuroimage55, 1816–1824 (2011). 10.1016/j.neuroimage.2011.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller, E. K. & Cohen, J. D. An Integrative Theory Of Prefrontal Cortex Function. www.annualreviews.org (2001). [DOI] [PubMed]
- 65.Boccia, M., Piccardi, L., Palermo, L., Nori, R. & Palmiero, M. Where do bright ideas occur in our brain? Meta-analytic evidence from neuroimaging studies of domain-specific creativity. Front. Psychol.6, 1195 (2015). [DOI] [PMC free article] [PubMed]
- 66.Choi, H. S., Seo, J. G., Hyun, J. & Bechtoldt, M. Collectivistic Independence Promotes Group Creativity by Reducing Idea Fixation. Small Group Res.50, 381–407 (2019). 10.1177/1046496419827990 [DOI] [Google Scholar]
- 67.Baer, J. & Kaufman, J. C. Gender differences in creativity. J. Creat. Behav. 42, 75–105. 10.1002/j.2162-6057.2008.tb01289.x (2008).
- 68.Abraham, A., Thybusch, K., Pieritz, K. & Hermann, C. Gender differences in creative thinking: Behavioral and fMRI findings. Brain Imaging Behav.8, 39–51 (2014). 10.1007/s11682-013-9241-4 [DOI] [PubMed] [Google Scholar]
- 69.Guilford, J. P., Christensen, P. R., Merrifield, P. R. & Wilson, R. C. Alternate uses. (1978).
- 70.Wang, X. et al. The contribution of divergent and convergent thinking to visual creativity. Think Skills Creat49, 101372 (2023).
- 71.Van Der Lee, C., Gatt, A., Van Miltenburg, E., Wubben, S. & Krahmer, E. Best practices for the human evaluation of automatically generated text. In Proceedings of the 12th International Conference on Natural Language Generation 355–368 (2019).
- 72.Agogué, M. et al. The impact of type of examples on originality: Explaining fixation and stimulation effects. J. Creative Behav.48, 1–12 (2014). 10.1002/jocb.37 [DOI] [Google Scholar]
- 73.Fahoum, N., Pick, H. & Shamay-Tsoory, S. The Impact of Creativity Training on Inter-Group Conflict-Related Emotions. J. Confl. Resolut.0, 1–28 (2023). [Google Scholar]
- 74.Torrance, E. P. Torrance tests of creative thinking: verbal tests, forms A and B; figural tests, forms A and B; norms-technical manual. (Personal Press, 1974).
- 75.Scholkmann, F. et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage85, 6–27 (2014). 10.1016/j.neuroimage.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 76.Hoshi, Y. Functional near-infrared spectroscopy: current status and future prospects. J. Biomed. Opt.12, 062106 (2007). 10.1117/1.2804911 [DOI] [PubMed] [Google Scholar]
- 77.Huppert, T. J., Diamond, S. G., Franceschini, M. A. & Boas, D. A. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt.48, 280–298 (2009). 10.1364/AO.48.00D280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yücel, M. A., Selb, J., Cooper, R. J. & Boas, D. A. Targeted principle component analysis: A new motion artifact correction approach for near-infrared spectroscopy. J. Innov. Opt. Health Sci.7, 1350066 (2014). [DOI] [PMC free article] [PubMed]
- 79.Yücel, M. A. et al. Best practices for fNIRS publications. Neurophotonics8, 012101 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wyatt, J. S., Delpy, D. T., Cope, M., Wray, S. & Reynolds, E. O. R. Quantification Of Cerebral Oxygenation And Haemodynamics In Sick Newborn Infants By Near Infrared Spectrophotometry. Lancet328, 1063–1066 (1986). 10.1016/S0140-6736(86)90467-8 [DOI] [PubMed] [Google Scholar]
- 81.Cui, X., Bray, S. & Reiss, A. L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage49, 3039–3046 (2010). 10.1016/j.neuroimage.2009.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Materials
Data Availability Statement
The datasets analyzed during this study is available at 10.5281/zenodo.12200031 (https://zenodo.org/records/12200032). The source data behind the graphs and table in the paper is available in Supplementary Data 1. Other data will be available from the corresponding author on reasonable request.
The codes generated during this study will be available from the corresponding author upon request.