Table 1.
Optimization of reaction conditionsa
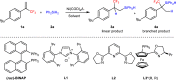 | ||||||
|---|---|---|---|---|---|---|
| Entry | L | L (%) | Solvent | T/(oC) | Yield (%) | L:B (3a:4a) |
| 1 | — | — | toluene | 30 | 18 | >20:1 |
| 2 | PPh3 | 10 mol% | toluene | 30 | 17 | >20:1 |
| 3 | PPh3 | 5 mol% | toluene | 30 | 96 | >20:1 |
| 4 | PCy3 | 5 mol% | toluene | 30 | 38 | >20:1 |
| 5 | BPY | 5 mol% | toluene | 30 | <5 | — |
| 6 | 1,10-phen | 5 mol% | toluene | 30 | <5 | 7:1 |
| 7b | L1 | 5 mol% | toluene | 30 | 81 | >20:1 |
| 8 | L2 | 5 mol% | toluene | 30 | 20 | >20:1 |
| 9 | L3 | 5 mol% | toluene | 30 | trace | — |
| 10 | BINAP | 8 mol% | toluene | 30 | NR | — |
| 11 | BINAP | 5 mol% | toluene | 30 | 96 | >1:20 |
| 12 | BINAP | 4.7 mol% | toluene | 30 | 96 | >1:20 |
| 13 | BINAP | 2.5 mol% | toluene | 30 | 90 | 1:9 |
| 14 | BINAP | 5 mol% | DMA | 30 | trace | — |
| 15 | BINAP | 5 mol% | MeOtBu | 30 | 59 | >1:20 |
| 16 | BINAP | 5 mol% | dioxane | 30 | 95 | >1:20 |
| 17 | BINAP | 5 mol% | DCE | 30 | NR | — |
| 18 | BINAP | 5 mol% | THF | 30 | <5 | — |
| 19 | BINAP | 5 mol% | EtOH | 30 | NR | — |
| 20 | BINAP | 5 mol% | toluene | 0 | NR | — |
| 21c | BINAP | 5 mol% | toluene | 30 | 60 | >1:20 |
| 22c | PPh3 | 5 mol% | toluene | 30 | 81 | >20:1 |
aReaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), Ni(cod)2 (5 mol%), L (x mol%), 30 oC in solvent (2.0 mL) under argon, 24 h, L:B value was determined by 19F NMR, isolated yield.
bCsOAc (20 mol%).
c2a (0.2 mmol).
