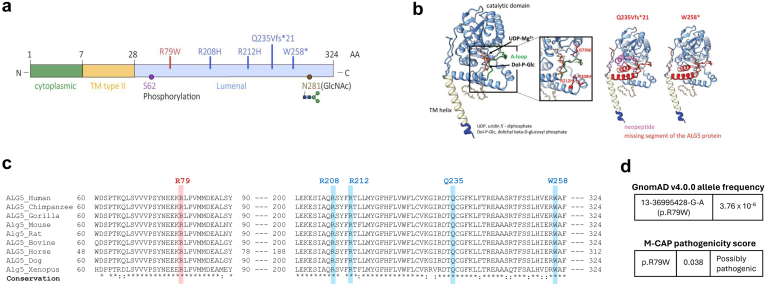
Figure 3.
In Silico structural mapping of missense variants in ALG5. (a) Diagram of the ALG5 sequence. Novel pathogenic variant identified in this study is shown in red. Previously reported dominant pathogenic variants associated with late-onset ADPKD are shown in blue. (b) Computational structural model of human dolichyl-phosphate beta-glucosyltransferase activity generated by AlphaFold (AF-Q9Y673-F1). Reaction products, dolichyl-phosphate (Dol-P) and Uridine 5'-diphospho (UDP)-alpha-D-glucose (UDP-Glc), are placed at the enzyme active site according to data from the crystal structure of archeal dolichyl-phosphate mannose synthase (see methods). Flexible region of the A-loop governing a dolichyl-phosphate beta-glucosyltransferase activity is highlighted in green. Mutated arginine residues previously reported (R208H, R212H) and the variant from this study (R79W) are shown as red sticks. The last 2 models represent mutants carrying premature stop codons. Missing parts of ALG5 protein and neopeptide in Gln235Valfs∗21 are highlighted by red and magenta, respectively. (c) Amino acid conservation across mutated segments of ALG5 in mammals. Asterisks (∗) indicate amino acid residues that are absolutely conserved and a colon (:) indicates residues with strong conservation across species. (d) Population allele frequency of R79W-ALG5 variant in GnomAD v4.0.0 database and computational prediction by Mendelian Clinically Applicable Pathogenicity Score (M-CAP) of the pathogenicity of missense R79W-ALG5 variant. ADPKD, autosomal dominant polycystic kidney disease.