Abstract
Introduction
Etelcalcetide is an i.v. calcimimetic agent, effectively reducing parathyroid hormone levels in patients on maintenance hemodialysis (HD). The clinical impact of discontinuing etelcalcetide at the time of kidney transplantation is unknown.
Methods
We retrospectively reviewed all patients on HD meeting predefined criteria who received a kidney transplant at our institution between January 1, 2015, and December 12, 2022. The incidence of parathyroidectomy and the evolution of calcium, phosphate, and intact parathyroid hormone (iPTH) levels after transplantation was analyzed according to the type of calcimimetic treatment before transplantation (cinacalcet vs. etelcalcetide vs. none).
Results
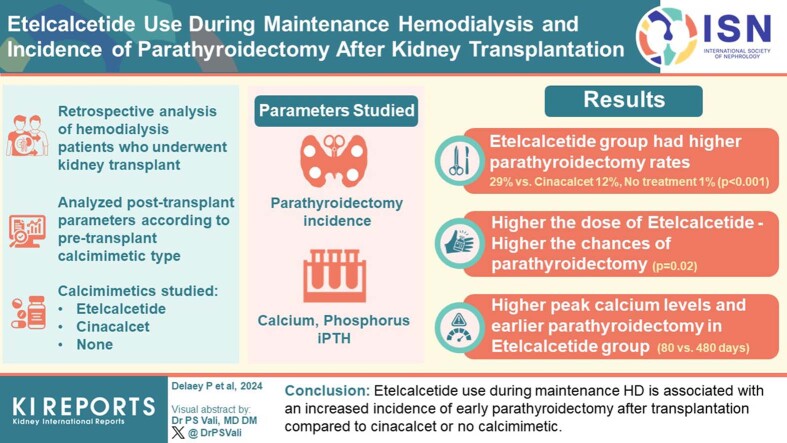
Overall, 372 patients (aged 53 years; interquartile range [IQR]: 42–62 years) were included. At the time of transplantation, 35, 75, and 262 patients were under etelcalcetide, cinacalcet, or no calcimimetic, respectively. After 1064 (IQR: 367–1658) days, the incidences of parathyroidectomy in the etelcalcetide, cinacalcet, no calcimimetic groups were 29%, 12%, and 1%, respectively (P < 0.001). Etelcalcetide was associated with an increased incidence of parathyroidectomy after adjustment for age, sex, and HD vintage (hazard ratio [HR]: 97.0, 95% confidence interval [CI]: 19.1–493.9, P < 0.001). The incidence of parathyroidectomy was related to etelcalcetide dosage (6/11 [54.6%] in patients with ≥ 10 mg vs. 4/24 [16.7%] in patients with < 10 mg, P = 0.02). Moreover, peak calcium levels were higher (P < 0.001) and parathyroidectomy was performed earlier (median 80 vs. 480 days, P < 0.001) in the etelcalcetide compared with the cinacalcet group. Long-term graft function, graft loss, and mortality were similar.
Conclusion
Etelcalcetide use during maintenance HD is associated with an increased incidence of early parathyroidectomy after transplantation compared to cinacalcet or no calcimimetic.
Keywords: chronic hemodialysis, etelcalcetide, hyperparathyroidism, kidney transplantation, mineral metabolism, parathyroidectomy
Graphical abstract
Secondary hyperparathyroidism (SHPT) is a frequent complication of chronic kidney disease, affecting most patients on maintenance dialysis.1 The use of calcimimetic agents has revolutionized the treatment of SHPT over the past 20 years.2 Cinacalcet was the first allosteric modulator of the calcium sensing receptor approved for SHPT treatment. Nevertheless, its use is fraught with relatively frequent side effects and, as a result, with suboptimal adherence.3 In 2017, etelcalcetide, an i.v. direct agonist of the calcium sensing receptor, was approved for the treatment of SHPT in adults undergoing HD,4 and is widely used.5 The administration of etelcalcetide at the end of each HD session largely overcomes the issue of suboptimal adherence with cinacalcet.6
Spontaneous correction of SHPT is uncommon shortly after successful kidney transplantation; regression of adaptative parathyroid gland hyperplasia is much slower than the recovery of kidney function.7, 8, 9, 10 Indeed, up to 42% of patients keep inappropriately high iPTH levels 3 months after transplantation,8 and persistent, maladaptive hyperparathyroidism has been documented in 17% to 50% of patients up to 2 years after successful transplantation.7 Hypercalcemia, usually mild, mostly resulting from persistent SHPT, is thus observed in up to 66% of incident transplant recipients and persists in about 30% of recipients at 1 year, with a progressive decrease thereafter.7,8 Some transplant patients may require parathyroidectomy for persistent SHPT with hypercalcemia.9,10 Cinacalcet use in patients on HD has been associated with an increased risk of posttransplantation parathyroidectomy.11,12 Whether etelcalcetide impacts the risk of posttransplantation parathyroidectomy has not been investigated. We previously reported acute and severe hypercalcemia early after kidney transplantation in 3 patients previously treated with high dose etelcalcetide, requiring urgent parathyroidectomy.13,14
The aim of this single-center retrospective study is to determine the incidence of posttransplantation parathyroidectomy according to the history and type of calcimimetic agent during maintenance HD (cinacalcet vs. etelcalcetide vs. no calcimimetic) in a cohort of kidney transplant recipients.
Methods
Patient Selection
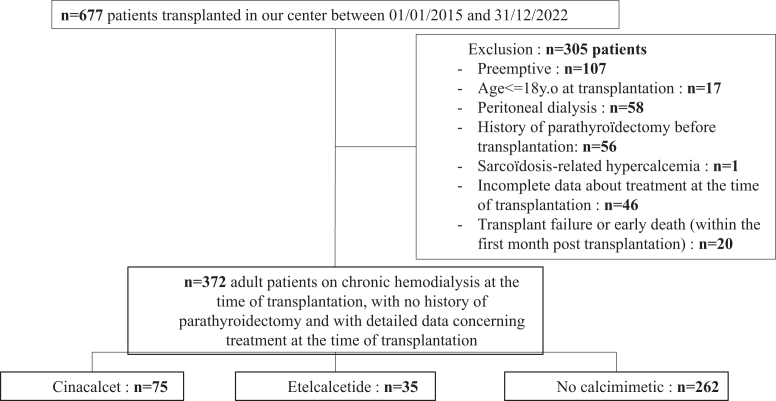
All adult patients followed-up with in a Belgian HD unit who received a kidney transplant in our center between January 1, 2015 and December 31, 2022 were reviewed. Considering that the main goal of the study was to assess the impact of etelcalcetide versus cinacalcet treatment before kidney transplantation on the incidence of posttransplant parathyroidectomy, we excluded patients receiving a preemptive transplantation and those on peritoneal dialysis, in whom etelcalcetide was not used. Patients with a history of pretransplant parathyroidectomy and patients with early graft failure or death (within the first month posttransplantation) were also excluded (Figure 1).
Figure 1.
Flow chart of the study.
The study population was split into 3 groups as follows: (i) the etelcalcetide group, (ii) the cinacalcet group, and (iii) a control group without calcimimetic at the time of transplantation (Figure 1). Data were collected until patient death, graft failure, or data collection cut-off on March 31, 2023.
The study adhered to the principles of the Declaration of Helsinki and was conducted after the approval of the UCLouvain-Cliniques universitaires Saint-Luc biomedical ethics committee (Reference 2022/22JULY/288). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Data Collection
The following data were collected: demographics (gender, age), dialysis vintage and last modality, cause of kidney failure, calcimimetic agent at the time of transplant (if any) and dosage, and transplantation data (date of transplantation, rank of transplantation, donor source, virtual panel reactive antibody, human leucocytes antigen number of mismatches, induction therapy, and maintenance immunosuppressive treatment). The use of other medications impacting bone metabolism (e.g., phosphate binders, calcium or vitamin D supplements, and diuretics) at the time of and/or after kidney transplantation was also collected. We also recorded the occurrence of delayed graft function (defined as the need for at least 1 HD session in the first week posttransplant for another reason than hyperkalemia) and of surgical parathyroidectomy and its timing after kidney transplantation.
Our center is a tertiary center for transplantation, collaborating closely with a network of nephrology centers in Belgium. To maximize the exhaustivity of clinical data (especially regarding the ongoing treatment at the time of transplantation), a standard form was sent to all nephrologists in charge of referred patients. The response rate was 93.2%.
Biochemical parameters included levels of plasma total calcium, phosphate, albumin, 25 hydroxyvitamin D, creatinine levels, and serum iPTH and 25-hydroxyvitamin D. All values were collected at different time points: on day 0 (day of transplantation), day 7, month 1, and month 3. Serum creatinine and estimated glomerular filtration rate levels at month 1, 3, and 12 were also collected.
All biochemical tests were performed in the biochemistry laboratory of Cliniques universitaires Saint-Luc. iPTH was measured by electrochemiluminescence (Roche diagnostics on Cobas e602 module; normal range 15–80 pg/ml) throughout the study period. Estimated glomerular filtration rate was estimated according to the 2009 Chronic Kidney Disease Epidemiology Collaboration equation.
Patient Management
Before transplantation, the decision to use a calcimimetic agent, when indicated during maintenance HD, was made by each dialysis center. In Belgium, etelcalcetide has been reimbursed for SHPT in HD since 2018. Our protocol for the management of kidney transplant recipients was not modified much during the study period. Induction therapy is not used in nonsensitized deceased donors and basiliximab is given in case of living donation. In sensitized patients (pretransplant panel reactive antibody >0%), plasma exchange therapy has been used for 1 month after transplantation until early 2020, and then replaced by Thymoglobulin (Sanofi, Belgium) because of the COVID-19 pandemic. All patients received a combination of tacrolimus, mycophenolate, and steroids as maintenance immunosuppression, cotrimoxazole for 6 months, and valganciclovir for 6 months (except in CMV D-/R-).
After discharge, all patients were followed-up with (including biochemical tests) at our outpatient clinic; twice a week for 2 months, then once a week for 1 month. They were subsequently followed-up with every 2 weeks in collaboration with the referring center until the end of the first year after transplantation.
The decision to perform parathyroidectomy after transplantation was made by the same medical team over the study period (AD, NK, AB, and MM), according to biochemical results (i.e., significant persistent or symptomatic hypercalcemia). Preoperative imaging included parathyroid glands ultrasound and scintigraphy. Parathyroidectomies were all performed by the same surgical team in our hospital.
In Belgium, calcimimetic use after kidney transplantation is virtually impossible. Indeed, cinacalcet is very expensive, is not reimbursed in this indication and a medical need program is not available. Etelcalcetide is not approved outside maintenance HD. Bisphosphonates and denosumab are not used to manage SHPT in transplanted patients in our center.
Outcomes
The main outcome of interest was posttransplant parathyroidectomy in the 3 study groups (cinacalcet vs. etelcalcetide vs. controls). Other studied outcomes were mineral metabolism parameters (calcium, phosphate, and iPTH) and allograft function (delayed graft function and estimated glomerular filtration rate 1 year after transplant). In the analysis of the evolution of biochemical parameters (plasma calcium and phosphate levels and iPTH levels) according to calcimimetic exposure, patients were censored after parathyroidectomy.
Statistics
Results are presented as median and IQR for continuous variables and as numbers and proportions for categorical variables. Comparisons between groups were performed using Kruskal Wallis, or chi-square test, as appropriate. Kaplan-Meier estimates and Cox proportional hazard regressions assessed the time to parathyroidectomy after kidney transplantation, considering the group of patients receiving no calcimimetic agent as the reference. The analysis was repeated after considering etelcalcetide use as the reference group. Adjusted analyses included the following prespecified covariates: age at the time of transplantation, gender, and dialysis vintage.15 Collinearity between variables was quantified using variance inflation factors. Statistical analyses were performed using Stata v17 (StataCorp, College Station, Texas, USA) and graphed with GraphPad Prism v19 (GraphPad Software, Boston, Massachusetts, USA). All tests were 2-tailed, and a P value < 0.05 was considered significant.
Results
Patient Selection and Baseline Characteristics
Among the 677 patients who received a kidney transplant in our center during the study period, 372 were included, 14 of which were previously on HD in Luxemburg a country neighboring Belgium. Forty six patients were excluded because of incomplete data on the treatment received during dialysis, and 259 patients were excluded according to the predefined exclusion criteria (Figure 1). Thirty five patients (9.4%), 75 patients (20%), and 262 patients (70.6%) were treated during maintenance HD with etelcalcetide, cinacalcet, or no calcimimetics, respectively (Supplementary Figure S2).
Table 1 summarizes the main baseline characteristics of the cohort. The median age at the time of transplantation was 53 (IQR: 42–62) years and was similar in the 3 groups. Although the time spent on dialysis was unsurprisingly longer for patients under calcimimetics compared to the control group, it was similar in the cinacalcet group versus etelcalcetide group (57 [IQR: 35–71] months vs. 69 [35–84] months). In addition, patients free of calcimimetic treatment at transplantation were more likely to receive a living donor transplantation (20%) compared to the other groups (8% and 9% in the cinacalcet group and the etelcalcetide group, respectively).
Table 1.
Baseline characteristics
| Variable | Whole cohort N = 372 |
No calcimimetic n = 262 | Cinacalcet n = 75 | Etelcalcetide n = 35 | P-value |
|---|---|---|---|---|---|
| Age, yr, median (IQR) | 53 (42–62) | 54 (43–63) | 52 (42–59) | 49 (40–65) | 0.62 |
| Female gender no. (%) | 127 (34) | 91 (35) | 19 (25) | 17 (49) | 0.05 |
| Kidney disease no. (%) | 0.85 | ||||
| Diabetes | 58 (16) | 41 (16) | 11 (15) | 6 (17) | |
| Glomerular disease | 96 (26) | 68 (26) | 22 (29) | 6 (17) | |
| Interstitial nephritis | 39 (10) | 29 (11) | 8 (11) | 2 (6) | |
| Hypertension/renal vascular disease | 20 (5) | 14 (5) | 3 (4) | 3 (9) | |
| Inherited | 83 (22) | 55 (21) | 19 (25) | 9 (26) | |
| Other | 26 (7) | 20 (8) | 2 (3) | 4 (11) | |
| Unknown | 50 (13) | 35 (13) | 10 (13) | 5 (14) | |
| Dialysis vintage, mo, median (IQR) | 39 (20–62) | 33 (17–52) | 57 (35–71) | 69 (35–84) | <0.001 |
| Treatment at the time of KT | |||||
| Ca-containing phosphate binder no. (%) | 231 (62) | 168 (64) | 42 (56) | 21 (60) | 0.40 |
| Non-Ca containing phosphate binder no. (%) | 177 (48) | 109 (42) | 47 (63) | 21 (60) | 0.002 |
| Vitamin D no. (%) | 238 (64) | 167 (64) | 48 (64) | 23 (66) | 0.98 |
| Active vitamin D no. (%) | 123 (33) | 77 (30) | 35 (47) | 11 (31) | 0.02 |
| Type of donor no. (%)a | 0.02 | ||||
| Deceased | 311 (84) | 210 (80) | 69 (92) | 32 (91) | |
| Living | 61 (16) | 52 (20) | 6 (8) | 3 (9) |
Ca, calcium; IQR, interquartile range; KT, kidney transplantation; iPTH, intact parathormone.
More data are available in Supplementary Table S2.
In the calcimimetic groups, patients were treated with cinacalcet or etelcalcetide for a median of 18.3 (IQR: 9.2–33) months and 18.3 (IQR: 6.3–23.8) months, respectively, before transplantation. Mean reported doses of cinacalcet and etelcalcetide were 53 ± 27 mg/d and 7 ± 2 mg/dialysis session, respectively.
Data regarding other baseline characteristics, including immunological data, are in the Supplementary Table S1).
Parathyroidectomy After Transplantation
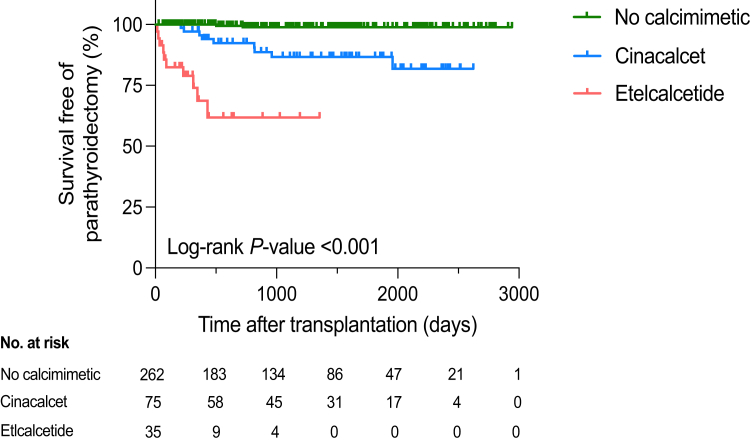
Patients were followed-up with for a median of 1064 (IQR: 367–1658) days after transplantation. Overall, 21 patients (6%) underwent parathyroidectomy, a median of 347 (IQR: 90–503) days posttransplantation. The incidence of parathyroidectomy was much higher in the etelcalcetide group (29%, n = 10) compared to 12% (n = 9) in the cinacalcet group and 1% (n = 2) in the control group) (P < 0.001). Time to parathyroidectomy after transplantation was shorter in the etelcalcetide group (median 80 [IQR: 42–292] days), both compared to the other groups (P < 0.001), or to the cinacalcet group only (median 480 [IQR: 364–819] days) (P < 0.001) (Table 3 and Figure 2). Of note, urgent parathyroidectomies performed during the hospitalization for kidney transplantation occurred only in etelcalcetide patients (4/10). Survival analysis using Cox proportional regressions confirmed the higher incidence of posttransplant parathyroidectomy in patients treated with etelcalcetide during maintenance HD, both in unadjusted (HR: 83.2; 95% CI: 17.7–391.2; P < 0.001) and adjusted (adjusted HR: 97.0; 95% CI: 19.1–493.9; P < 0.001) analyses (Table 2). When the etelcalcetide group was considered as the reference, cinacalcet use had a protective impact regarding parathyroidectomy, both in unadjusted (HR, 0.19; 95% CI: 0.73–0.5; P < 0.001) and adjusted (adjusted HR: 0.2; 95% CI: 0.08–0.51; P < 0.001) analyses (Table 2).
Table 3.
Outcomes after transplantation
| Outcome | Whole cohort N = 372 |
No calcimimetic n = 262 | Cinacalcet n = 75 | Etelcalcetide n = 35 | P-value |
|---|---|---|---|---|---|
| Follow-up after transplantation, d, median (IQR) | 1064 (367–1658) | 1078 (377–1652) | 1464 (728–1980) | 349 (241–646) | <0.001 |
| Parathyroidectomy after transplantation, no. (%) | 21 (6) | 2 (1) | 9 (12) | 10 (29) | <0.001 |
| Time to parathyroidectomy after transplantation, d, median (IQR) | 347 (90–503) | 612 (503–721) | 480 (364–819) | 80 (42–292) | <0.001 |
| Delayed graft function, no. (%) | 52 (14) | 29 (11) | 15 (21) | 8 (23) | 0.04 |
| Delayed graft functiona, no. (%) | 51/309 (17) | 28/210 (13) | 15/67 (22) | 8/32 (25) | 0.09 |
| Loss of kidney allograft, no. (%) | 15 (4) | 12 (5) | 3 (4) | 0 (0) | 0.43 |
| Death no. (%) | 37 (10) | 28 (11) | 7 (9) | 2 (6) | 0.64 |
| Max. calcium level, mMmol/l, median (IQR) | 2.58 (2.51–2.67) | 2.56 (2.49–2.62) | 2.63 (2.58–2.75) | 2.81 (2.60–2.99) | <0.001 |
| Time to max. calcium level, d, median (IQR) | 102 (48–201) | 111 (49–187) | 112 (61–229) | 52 (29–157) | 0.02 |
| eGFR at 12 mo, ml/min per 1.73 m2, median (IQR) | 56 (45–71) | 56 (45–70) | 54 (43–71) | 66 (53–85) | 0.05 |
eGFR, estimated glomerular filtration rate; IQR, interquartile range; max., maximal; no.; number.
In patients with a deceased donor kidney transplantation.
Figure 2.
Survival free of parathyroidectomy according to calcimimetic exposure (Kaplan-Meier analysis).
Table 2.
Cox proportional hazards model for time to parathyroidectomy after kidney transplantation according to pretransplant calcimimetic use
| Unadjusted model |
Adjusted model |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P-value | |
| No calcimimetic | 1.0 (ref.) | 1.0 (ref.) | ||||
| Cinacalcet | 14.4 | 3.1–66.5 | 0.001 | 14.2 | 3.0–67.9 | 0.001 |
| Etelcalcetide | 83.2 | 17.7–391.2 | <0.001 | 97.0 | 19.1–493.9 | <0.001 |
| Etelcalcetide | 1.0 (ref.) | 1.0 (ref.) | ||||
| Cinacalcet | 0.19 | 0.073–0.5 | <0.001 | 0.2 | 0.08–0.51 | <0.001 |
95% CI, 95% confidence interval; HR, hazard ratio.
Adjusted for age at the time of transplantation, gender, and dialysis vintage.
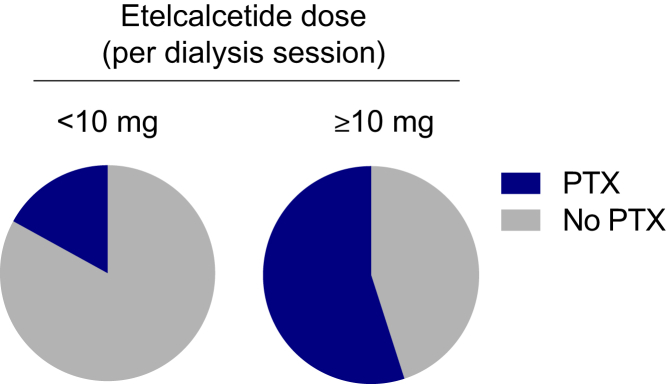
Supplementary Table S2 shows the distribution of the incidence of parathyroidectomy in the etelcalcetide group according to the last dosage received during HD. In patients under etelcalcetide ≥10 mg/dialysis session (n = 11), the incidence of parathyroidectomy was 54.6% (n = 6), compared to 16.7% (n = 4) in patients under a lower dosage (n = 24) (P = 0.02) (Figure 3).
Figure 3.
Distribution of the incidence of parathyroidectomy in the etelcalcetide group according to the last dosage (<10 mg vs. ≥10 mg/dialysis session) received during maintenance hemodialysis. PTX, parathyroidectomy.
After parathyroidectomy, no wound bleeding, infection, or laryngeal recurrent nerve palsy were observed. Hypocalcemia related to hungry bone syndrome after parathyroidectomy occurred in 50% and 67% of etelcalcetide and cinacalcet patients, respectively (P = 0.65), without requiring intensive care or rehospitalization.
Evolution of Calcium, Phosphate and iPTH Levels After Transplantation
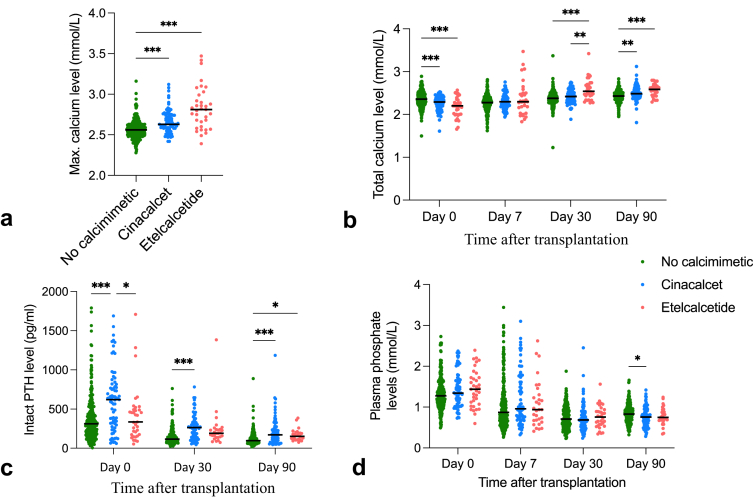
As shown in Table 3 and Figure 4a, patients in the etelcalcetide group had higher peak levels of plasma calcium after transplantation than the other groups (2.81 [IQR: 2.60–2.99] mMol/l vs. 2.63 [IQR: 2.58–2.72] mMol/l in the cinacalcet group and 2.56 [IQR: 2.49–2.62] mMol/l in the control group, P < 0.001). The plasma calcium peak was reached earlier in the etelcalcetide group (52 [IQR: 29–157] days) compared with 112 (IQR: 61–229) days in the cinacalcet group, and 111 (IQR: 49–187) days in the control group (P = 0.02). Similarly, in patients reaching hypercalcemia >2.55 mMol/l, the time to this cut-off was shorter in the etelcalcetide group (21 [IQR: 11.5–60) days]) than in the cinacalcet and control groups (48.5 [IQR: 19–159] and 47.5 [IQR: 19.5–109.5] days, respectively) (P = 0.01).
Figure 4.
Evolution of biochemical parameters after kidney transplantation, according to calcimimetic exposure. Horizontal lines represent mean values. Significance level is denoted by asterisks (P < 0.05; P < 0.01; P < 0.001). (a) Maximal plasma calcium level. (b) Total plasma calcium level. (c) Serum intact PTH level. (d) Plasma phosphate level.
The evolution of plasma calcium, phosphate, and serum iPTH (censored for parathyroidectomy, if any) values differed between groups (Figure 4b and d). At baseline (day 0, just before transplantation), plasma calcium level was lower in both calcimimetic groups than in the control group (Figure 4b). Remarkably, patients under cinacalcet had the highest levels of serum iPTH compared to the 2 other groups at baseline (Figure 4c). Compared to day 0, iPTH values decreased on day 30 in all groups but were higher in both calcimimetic groups than in the control group. Of note, although iPTH values were similar in the cinacalcet and etelcalcetide groups at this time point, plasma calcium levels were significantly higher in the etelcalcetide group than in the 2 other groups. Three months after transplantation, both calcium and iPTH levels were similar in the cinacalcet and etelcalcetide groups but remained significantly higher in comparison with the control group. Plasma phosphate levels already decreased 1 week after transplantation and remained similar between the 3 groups at all time points (Figure 4d). At the time of parathyroidectomy, plasma calcium levels were significantly higher in etelcalcetide than in cinacalcet patients (2.87 [IQR: 2.76–2.97] mMol/l vs. 2.73 [2.65–2.82] mMol/l, respectively [P = 0.03]). iPTH levels were higher in etelcalcetide compared with cinacalcet patients, however not reaching significance (244 [IQR: 186–507] pMol/ml vs. 194 [IQR: 112–368] pMol/ml, respectively [P = 0.34]) (Supplementary Figure S1).
Allograft and Patient Outcomes
After a median follow up of 1064 (IQR: 367–1658) days, the incidence of allograft loss and patient death was similar in all groups (Table 3). We did not observe a difference in allograft function in the first-year posttransplant. Interestingly, the incidence of delayed graft function tended to be higher in patients treated with a calcimimetic agent (cinacalcet or etelcalcetide) compared than in the control group (Table 3). Because the rate of living donor transplantation was higher in the control group (Table 1), we repeated the analysis including only patients who received a deceased donor transplantation. Although the difference persisted (25% in the etelcalcetide group and 22% in the cinacalcet group vs. 13% in the control group), it was not statistically significant (P = 0.09).
Discussion
Our study shows that etelcalcetide use during maintenance HD is associated with an increased incidence of early parathyroidectomy after kidney transplantation compared to cinacalcet treatment (29% vs. 12%). This resulted from the fact that patients in the etelcalcetide group showed a greater and much faster increase of plasma calcium level after transplantation, compared to the other groups, despite the fact that, at the time of transplantation, calcemia was similar in both calcimimetic groups and iPTH levels were even higher in cinacalcet compared with etelcalcetide patients. The role of etelcalcetide as a risk factor for early parathyroidectomy is further supported by the dose-dependent relationship with etelcalcetide dosage, the incidence of parathyroidectomy being 54.6% in those under etelcalcetide ≥10 mg/d.
Our 29% rate of early parathyroidectomy after transplantation is dramatically higher than figures published before etelcalcetide availability. An overall parathyroidectomy rate of 5.2% was reported in a case series of 1743 patients transplanted between 1989 and 2004.8 Another case series of 990 patients transplanted between 2003 and 2008 showed a 1.5% rate of posttransplant parathyroidectomy.10 In a nested case control study including 100 kidney transplant recipients (31 of whom had hypercalcemia), Nanmoku et al.12 documented an overall incidence of posttransplant parathyroidectomy of 3%; moreover, cinacalcet use before transplantation was identified as a significant independent risk factor for hypercalcemia after transplantation. Similarly, pretransplant cinacalcet treatment was associated with higher posttransplant PTH and serum calcium levels and more prevalent nephrocalcinosis 3 months after kidney transplantation, in comparison with cinacalcet naïve controls.11
Interestingly, Evenepoel et al.11 reported a 28.6% rate of parathyroidectomy in 21 kidney transplant recipients treated with cinacalcet before transplantation, 6.6 (3.8–11.2) months after transplantation, compared with 6.7% in 282 transplanted controls (P = 0.0005). The fact that our incidence rate of parathyroidectomy after etelcalcetide use is similar to that observed in the above mentioned study after cinacalcet use may suggest a lower adherence to oral cinacalcet in patients included in the present study. Indeed, the etelcalcetide group had a much better biochemical control of SHPT (lower calcium and iPTH levels) at day 0 (just before transplantation) compared to the cinacalcet group. This is in line with trials showing that etelcalcetide is associated with greater reductions in iPTH and calcium levels in dialysis patients with SHPT, as compared to cinacalcet, despite similar pharmacodynamic properties.16,17 This is most likely explained by the suboptimal adherence tolerance of patients to cinacalcet, as already demonstrated by clinical trials18 and real world data.3 In contrast, patient’s adherence to etelcalcetide is much better, almost “guaranteed” by the i.v. administration of the drug at each HD session.16 Thus, it seems reasonable to hypothesize that etelcalcetide allows easier biochemical control of severe SHPT in maintenance HD, that might have required parathyroidectomy after cinacalcet failure. Etelcalcetide abrupt withdrawal at the time of transplantation, together with a rapid renal clearance by a functional kidney transplant19,20 might lead to a flare up of unmasked SHPT, triggering the rapid posttransplant hypercalcemia development, potentially through the response of the kidney allograft to the hyperactivated PTH calcitriol axis as well as through its participation in the PTH-independent tight calcium control mechanisms.
Our study suggests reconsidering the current practice in the management of patients with SHPT on the waiting list for kidney transplantation. Indeed, relevant published guidelines all recommend considering parathyroidectomy before transplantation in case of severe SHPT when medical treatments fail, without clear indications on optimal PTH targets or the timing of parathyroidectomy.21, 22, 23, 24 Moreover, the decision to perform posttransplant parathyroidectomy is difficult, and guidelines are currently lacking. Our data suggest that etelcalcetide use, especially at high dosages, should be incorporated in the decision making to consider parathyroidectomy before transplantation even if SHPT is biochemically controlled to avoid early SHPT rebound after transplantation.25 Interestingly, calcimimetics withdrawal for 2 to 4 weeks before measuring iPTH has been suggested, together with assessment of parathyroid glands size, to assess the real severity of SHPT, and thus the potential need for pretransplantation parathyroidectomy, in kidney transplant candidates.25
Previous studies have shown that the association between posttransplantation hypercalcemia and cinacalcet received before transplantation is dose-dependent.11,26 The number of patients included in the etelcalcetide group in our study is too small to determine a robust dosage cut-off of etelcalcetide to consider pretransplant parathyroidectomy. Nevertheless, considering our data, it seems reasonable to avoid dosages ≥10 mg in patients on HD on the waiting list. Taken together, these data suggest that some patients receiving etelcalcetide for SHPT during maintenance HD might benefit from parathyroidectomy before transplantation to avoid severe hypercalcemia requiring early parathyroidectomy after transplantation. This may be even more true for etelcalcetide and should be investigated further.
We acknowledge several limitations mostly related to the retrospective and monocentric design of the study. However, the protocol of follow-up of kidney transplant recipients at our outpatient clinic did not change much during the study period and all laboratory tests were performed in our university hospital. Furthermore, despite the exclusion of a number of patients due to incomplete data concerning treatment before transplantation, the decision of parathyroidectomy has been made centrally at our institution by the same medical staff and is thus unlikely to have changed over the period 2015 to 2022, which makes the rate of parathyroidectomy a robust parameter. Moreover, at the time of parathyroidectomy, plasma calcium levels were significantly higher in etelcalcetide patients than in the cinacalcet patients, despite similar calcium levels at the time of transplantation; a strong argument against a potential indication bias favoring earlier parathyroidectomy in patients previously treated by etelcalcetide. Third, the number of etelcalcetide patients was small, making the comparison with the cinacalcet group difficult to interpret, as shown by the large 95% CI of the Cox regression analysis. However, both calcimimetic groups were quite similar concerning not only time on dialysis (a key risk factor for SHPT15), but also time on calcimimetic treatment, almost identical in both groups (18 months), which makes the hypothesis of a more severe SHPT in patients started with etelcalcetide very unlikely. Moreover, due of the better adherence, etelcalcetide has largely replaced cinacalcet use after its commercial availability in Belgium in 2018. Thus, both cohorts of calcimimetic patients are largely consecutive rather than simultaneous (Supplementary Figure S2) and the choice between cinacalcet or etelcalcetide is thus not expected to be based on the severity of SHPT. Nevertheless, should some patients in the cinacalcet group receive some tablets of cinacalcet after kidney transplantation because of hypercalcemia (i.e., until finishing the last 28 tablet box started before transplantation), this would not be expected to significantly influence the large difference in time to parathyroidectomy observed between cinacalcet and etelcalcetide patients (480 vs. 80 days, respectively). Fourth, clinical long term outcomes such as fracture rates and bone density parameters were not measured. Lastly, this study did not allow to depict some of the characteristics of maladaptive, persistent SHPT after kidney transplantation, that is, hypocalciuria.27
It is tempting to speculate that an early posttransplantation use of cinacalcet in patients previously treated with etelcalcetide would have reduced the incidence of parathyroidectomy. However, some limitations concerning the use of cinacalcet after kidney transplantation must be pointed out, including the cost and the risk of drug-drug interaction.28 Moreover, the efficacy of cinacalcet after transplantation on parathyroidectomy incidence is yet to be demonstrated. In the only published randomized controlled trial assessing the use of cinacalcet post–kidney transplantation versus placebo, cinacalcet has been shown to normalize mineral metabolism parameters after transplantation but its impact on bone density has been very limited.29 Similarly, 2 small studies including kidney transplant patients with persistent HPT and hypercalcemia showed that cinacalcet use was associated with smaller reductions of calcium and PTH concentrations compared with parathyroidectomy but without improvement of bone density (unlike parathyroidectomy).30,31 Furthermore, although parathyroidectomy is invasive and has been associated with perisurgical and postsurgical risks,25 a successful parathyroidectomy permanently corrects maladaptive SHPT whereas rebound SHPT may develop after cinacalcet withdrawal.27
Of note, off-label treatment with cinacalcet is often used in some countries in order to bridge posttransplant-SHPT during the first months after transplantation. Our study suggests that, in such countries, physicians should consider pretransplant parathyroidectomy individually in those patients who may be most vulnerable to uncontrolled posttransplant SHPT, that is, patients treated with etelcalcetide because of an intolerance to cinacalcet.
In conclusion, our study shows that etelcalcetide use during maintenance HD is associated with an increased incidence of early parathyroidectomy after kidney transplantation, especially in those under high dosage (≥ 10 mg) etelcalcetide, of which more than 50% required early parathyroidectomy. Those patients may deserve a close follow-up of calcium and iPTH levels in the early course after transplantation. Further large scale studies should focus on the optimal management of patients treated with etelcalcetide awaiting a kidney transplant.
Appendix
List of Members of Kidney Transplantation Network Collaborators
Pierre -Yves Decleire, Service de néphrologie, Clinique Sud Luxembourg, Arlon, Belgium.
Marie Rommelaere, Service de néphrologie, Hôpital Princesse Fabiola, Aye, Belgium.
Miguel-Ange Guillen, Service de néphrologie, CHR Epicura, Mons, Belgium.
Benoit Buysschaert, Service de néphrologie, CHR de Huy, Huy, Belgium.
Bénédicte Vanderperren, Service de néphrologie, Centre hospitalier de Jolimont, La Louvière, Belgium.
Charles Cuvelier, Service de néphrologie, CHU UCL -Namur – Site Ste Elisabeth, Namur, Belgium.
Benoît Georges, Service de néphrologie, CHR Sambre -Meuse (Namur), Namur, Belgium.
Eugenia Papakrivopoulou, Service de néphrologie, Clinique Saint -Jean, Brussels, Belgium.
Claude Braun, Service de néphrologie, Hôpitaux Robert Schuman, Luxembourg.
Gaëlle Gillerot, Service de néphrologie, Clinique Saint -Pierre, Ottignies, Belgium.
Jean-Philippe Lengelé, Service de néphrologie, Grand Hôpital de Charleroi, Charleroi, Belgium.
François Reginster, Service de néphrologie, Cliniques de l’Europe, Brussels, Belgium.
Philippe Leroy, Service de néphrologie, Hôpital de Mons – Groupe Jolimont, Mons, Belgium.
Ann-Karolien Vandooren, Service de néphrologie, CH de Mouscron, Mouscron, Belgium.
Philippe Madhoun, Service de néphrologie, Centre Hospitalier de Wallonie picarde, Tournai, Belgium.
Disclosure
AD reports consultancy fees from Alnylam and Merck, outside the submitted work. JM reports consultancy fees for Alexion Pharmaceuticals, AstraZeneca, Bayer, GlaxoSmithKline, and Sanofi-Genzyme; research funding from Alexion Pharmaceuticals, AstraZeneca, and Baxter Healthcare; advisory or leadership roles for Alexion Pharmaceuticals, AstraZeneca, Bayer, Sanofi-Genzyme, and Versantis; speaker honoraria for AstraZeneca, Baxter Healthcare, and Fresenius Medical Care; funding from the National Fund for Scientific Research (FRS-FNRS, Brussels, Belgium), the Association pour l’Information et la Recherche sur les Maladies Rénales Génétiques (AIRG, Brussels, Belgium), and the Saint-Luc Foundation (Brussels, Belgium); and travel support from Sanofi-Genzyme and Vifor Pharma. ND reports consultancy fees from Hansa Biopharma, Pfizer, Takeda, and Astellas, outside the submitted work. TD reports research support from the Fonds National de Recherche Scientifique – FNRS (F.R.S.-FNRS), and travel support from Organ Recovery Systems, Diegem, Belgium, outside the submitted work. EG reports research support from Baxter Healthcare; conference support from Baxter Healthcare, Fresenius, Nx Stage, Dirinco, and AstraZeneca; consulting fees from Amgen, Astellas, AstraZeneca, Bayer, Baxter Healthcare, Fresenius, NxStage, and Dirinco; none of those related to the present study. MJ reports research support from AstraZeneca, speaker fees from AstraZeneca, Bayer, and Boehringer-Ingelheim; consulting fees from Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiorenal, CSL Vifor, GSK, Stada-Eurogenerics, and Vertex; and co-chair of Kidney Disease Improving Global Outcomes (KDIGO) since January 2019; all outside the submitted work. LL reports conference honoraria from Nipro, Amgen, and Vifor Med care Pharma; travel support from Bayer, Sanofi-Genzyme and Vifor Med care Pharma; and consultancy fees from Vifor Med care Pharma, Pharmanovia, and Hemotech, outside the submitted work. All the authors declared no competing interests.
Acknowledgements
This work was presented at the annual congress of the American Society of Nephrology (Philadelphia, November 2023) and the Société Française de Néphrologie, Dialyse et Transplantation (Liège, October 2023). We sincerely thank the patients and the clinicians for their contributions to this study.
Author Contributions
PD, AD, MJ, and LL were involved in the research idea, study design, data analysis, writing of the manuscript. PD, DF, and MI did data acquisition; JM did the statistical analysis. All authors participated in the critical writing and approved the final version of the manuscript. All co-authors were actively involved in the retrospective identification and recruitment of patients.
Footnotes
Figure S1. Patients started on cinacalcet or etelcalcetide over the study period.
Figure S2. Plasma calcium and iPTH levels at the time of parathyroidectomy in cinacalcet and etelcalcetide patients.
Table S1. Supplemental baseline characteristics.
Table S2. Dose of etelcalcetide and occurrence of parathyroidectomy after kidney transplantation.
Contributor Information
Laura Labriola, Email: laura.labriola@saintluc.uclouvain.be.
CUSL Kidney Transplantation Network Collaborators:
Pierre-Yves Decleire, Marie Rommelaere, Miguel-Ange Guillen, Benoit Buysschaert, Bénédicte Vanderperren, Charles Cuvelier, Benoît Georges, Eugenia Papakrivopoulou, Claude Braun, Gaëlle Gillerot, Jean-Philippe Lengelé, François Reginster, Philippe Leroy, Ann-Karolien Vandooren, and Philippe Madhoun
Supplementary Material
Figure S1. Patients started on cinacalcet or etelcalcetide over the study period. Figure S2. Plasma calcium and iPTH levels at the time of parathyroidectomy in cinacalcet and etelcalcetide patients. Table S1. Supplemental baseline characteristics. Table S2. Dose of etelcalcetide and occurrence of parathyroidectomy after kidney transplantation.
References
- 1.Hannan F.M., Kallay E., Chang W., Brandi M.L., Thakker R.V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat Rev Endocrinol. 2018;15:33–51. doi: 10.1038/s41574-018-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Block G.A., Martin K.J., de Francisco A.L., et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 3.Fuller D.S., Hallett D., Dluzniewski P.J., et al. Predictors of Cinacalcet discontinuation and reinitiation in hemodialysis patients: results from 7 European countries. BMC Nephrol. 2019;20:169. doi: 10.1186/s12882-019-1355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block G.A., Bushinsky D.A., Cunningham J., et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317:146–155. doi: 10.1001/jama.2016.19456. [DOI] [PubMed] [Google Scholar]
- 5.Karaboyas A., Muenz D., Fuller D.S., et al. Etelcalcetide utilization, dosing titration, and chronic kidney disease-mineral and bone disease (CKD-MBD) marker responses in US hemodialysis patients. Am J Kidney Dis. 2022;79:362–373. doi: 10.1053/j.ajkd.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Arenas M.A., Rodelo-Haad C., Pendón-Ruiz de Mier M.V., Rodriguez M. Control of hyperparathyroidism with the intravenous calcimimetic etelcalcetide in dialysis patients adherent and non-adherent to oral calcimimetics. Clin Kidney J. 2020;14:840–846. doi: 10.1093/ckj/sfaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evenepoel P., Claes K., Kuypers D., Maes B., Bammens B., Vanrenterghem Y. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant. 2004;19:1281–1287. doi: 10.1093/ndt/gfh128. [DOI] [PubMed] [Google Scholar]
- 8.Evenepoel P., Van Den Bergh B., Naessens M., et al. Calcium metabolism in the early posttransplantation period. Clin J Am Soc Nephrol. 2009;4:665–672. doi: 10.2215/CJN.03920808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evenepoel P., Claes K., Kuypers D.R., Debruyne F., Vanrenterghem Y. Parathyroidectomy after successful kidney transplantation: a single centre study. Nephrol Dial Transplant. 2007;22:1730–1737. doi: 10.1093/ndt/gfm044. [DOI] [PubMed] [Google Scholar]
- 10.Muirhead N., Zaltman J.S., Gill J.S., et al. Hypercalcemia in renal transplant patients: prevalence and management in Canadian transplant practice. Clin Transpl. 2014;28:161–165. doi: 10.1111/ctr.12291. [DOI] [PubMed] [Google Scholar]
- 11.Evenepoel P., Sprangers B., Lerut E., et al. Mineral metabolism in renal transplant recipients discontinuing Cinacalcet at the time of transplantation: a prospective observational study. Clin Transpl. 2012;26:393–402. doi: 10.1111/j.1399-0012.2011.01524.x. [DOI] [PubMed] [Google Scholar]
- 12.Nanmoku K., Shinzato T., Kubo T., Shimizu T., Yagisawa T. Prevalence and predictors of early hypercalcemia after kidney transplantation: a nested case-control study within a cohort of 100 patients. Clin Exp Nephrol. 2019;23:268–274. doi: 10.1007/s10157-018-1627-6. [DOI] [PubMed] [Google Scholar]
- 13.Dachy G., Pochet J.M., Labriola L., et al. Severe hypercalcaemia early after kidney transplantation in two patients with severe secondary hyperparathyroidism previously treated with etelcalcetide. Clin Kidney J. 2021;14:1977–1979. doi: 10.1093/ckj/sfab063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foguenne M., Mourad M., Buemi A., et al. Acute and severe hypercalcemia early after kidney transplantation in a patient previously treated with etelcalcetide. Transpl Int. 2023;36 doi: 10.3389/ti.2023.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young E.W., Albert J.M., Satayathum S., et al. Predictors and consequences of altered mineral metabolism: the dialysis outcomes and practices patterns study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 16.Block G.A., Bushinsky D.A., Cheng S., et al. Effect of etelcalcetide vs Cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA. 2017;317:156–164. doi: 10.1001/jama.2016.19468. [DOI] [PubMed] [Google Scholar]
- 17.Eidman K.E., Wetmore J.B. Treatment of secondary hyperparathyroidism: how do cinacalcet and etelcalcetide differ? Semin Dial. 2018;31:440–444. doi: 10.1111/sdi.12734. [DOI] [PubMed] [Google Scholar]
- 18.EVOLVE Trial Investigators. Chertow G.M., Block G.A., et al. Effect of Cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 19.Shen J., Xiao J., Pickthorn K., et al. A pharmacokinetic/pharmacodynamic model for AMG 416, a novel calcimimetic peptide, following a single intravenous dose in healthy subjects. J Clin Pharmacol. 2014;54:1125–1133. doi: 10.1002/jcph.314. [DOI] [PubMed] [Google Scholar]
- 20.Wu B., Melhem M., Subramanian R., et al. Clinical pharmacokinetics and pharmacodynamics of etelcalcetide, a novel calcimimetic for treatment of secondary hyperparathyroidism in patients with chronic kidney disease on hemodialysis. J Clin Pharmacol. 2018;58:717–726. doi: 10.1002/jcph.1090. [DOI] [PubMed] [Google Scholar]
- 21.Kasiske B.L., Cangro C.B., Hariharan S., et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant. 2001;1(2):S3–S95. [PubMed] [Google Scholar]
- 22.Knoll G., Cockfield S., Blydt-Hansen T., et al. Canadian society of transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173:S1–S25. doi: 10.1503/cmaj.1041588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abramowicz D., Cochat P., Claas F.H., et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant. 2015;30:1790–1797. doi: 10.1093/ndt/gfu216. [DOI] [PubMed] [Google Scholar]
- 24.Chadban S.J., Ahn C., Axelrod D.A., et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020;104:S11–S103. doi: 10.1097/TP.0000000000003136. [DOI] [PubMed] [Google Scholar]
- 25.Cianciolo G., Tondolo F., Barbuto S., et al. A roadmap to parathyroidectomy for kidney transplant candidates. Clin Kidney J. 2022;15:1459–1474. doi: 10.1093/ckj/sfac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torregrosa J.V., Bergua C., Martinez de Osaba M.J., Oppenheimer F., Campistol J.M. Evolution of secondary hyperparathyroidism after kidney transplantation in patients receiving cinacalcet on dialysis. Transplant Proc. 2009;41:2396–2398. doi: 10.1016/j.transproceed.2009.06.073. [DOI] [PubMed] [Google Scholar]
- 27.Jørgensen H.S., Evenepoel P. Persistent hyperparathyroidism: a reality calling for additional evidence. Am J Kidney Dis. 2023;81:256–258. doi: 10.1053/j.ajkd.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Kilford P., Khoshaein N., Southall R., Gardner I. Physiologically-based pharmacokinetic models of CYP2D6 substrate and inhibitors nebivolol, cinacalcet and mirabegron to simulate drug-drug interactions. Eur J Drug Metab Pharmacokinet. 2022;47:699–710. doi: 10.1007/s13318-022-00775-8. [DOI] [PubMed] [Google Scholar]
- 29.Evenepoel P., Cooper K., Holdaas H., et al. A randomized study evaluating Cinacalcet to treat hypercalcemia in renal transplant recipients with persistent hyperparathyroidism. Am J Transplant. 2014;14:2545–2555. doi: 10.1111/ajt.12911. [DOI] [PubMed] [Google Scholar]
- 30.Cruzado J.M., Moreno P., Torregrosa J.V., et al. A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. J Am Soc Nephrol. 2016;27:2487–2494. doi: 10.1681/ASN.2015060622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung S., Kim H., Kwon H., et al. Parathyroidectomy versus cinacalcet in the treatment of tertiary hyperparathyroidism after kidney transplantation: a retrospective study. Kidney Res Clin Pract. 2022;41:473–481. doi: 10.23876/j.krcp.21.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patients started on cinacalcet or etelcalcetide over the study period. Figure S2. Plasma calcium and iPTH levels at the time of parathyroidectomy in cinacalcet and etelcalcetide patients. Table S1. Supplemental baseline characteristics. Table S2. Dose of etelcalcetide and occurrence of parathyroidectomy after kidney transplantation.