Abstract
Livedoid vasculopathy (LV) is a chronic, recurrent thrombotic vasculopathy characterized by painful ulcerations on the lower extremities, which heal slowly and leave atrophic white scars known as “atrophie blanche.” This report presents the case of a 31-year-old woman with a 4-year history of recurrent painful ulcerations on her legs and feet. A skin biopsy revealed findings consistent with LV, and an exhaustive laboratory workup ruled out secondary causes such as thrombophilia, malignancies, autoimmune diseases, and peripheral arterial disease. The patient showed remarkable improvement with a treatment regimen of pentoxifylline, nifedipine, and warfarin, resulting in complete ulcer resolution and sustained remission over 5 months. Our case highlights the importance of a comprehensive diagnostic approach and a multidisciplinary treatment strategy in managing primary LV to achieve remission and prevent recurrence of skin ulcerations.
Keywords: livedoid vasculopathy, atrophie blanche, ulceration, warfarin, pentoxifylline, nifedipine
Key Message
Livedoid vasculopathy has a wide set of differentials, including thrombophilia, malignancies, autoimmune diseases, and peripheral arterial disease. In cases of primary disease like this one, a combination of pentoxifylline, nifedipine, and warfarin was required to achieve remission and prevent skin ulceration.
Introduction
Livedoid vasculopathy (LV) is a rare, chronic vascular disorder primarily affecting the microcirculation of the skin, particularly on the lower extremities. Characterized by painful, recurrent ulcerations and a distinctive net-like purplish discoloration known as livedo racemose, LV poses significant diagnostic and therapeutic challenges. The healing of these ulcers typically results in white, atrophic scars called “atrophie blanche.” 1
The pathogenesis of LV is not fully understood, but it is believed to involve abnormalities in coagulation and fibrinolysis pathways, leading to the occlusion of dermal blood vessels by fibrin thrombi. This vascular occlusion results in localized ischemia and tissue necrosis, which are key features of the disease. Livedoid vasculopathy can be associated with various systemic conditions, including thrombophilia, autoimmune disorders, and connective tissue diseases, suggesting a multifactorial etiology with both thrombotic and inflammatory components. 2
Patients with LV often have a long history of painful, non-healing ulcers on the lower legs and feet. These ulcers are resistant to conventional wound care and can severely affect quality of life due to chronic pain and disability. Diagnosis requires a combination of clinical evaluation, histopathological examination, and extensive laboratory workup to exclude other potential causes such as hematologic malignancies and autoimmune diseases. Histopathologically, LV is identified by the presence of fibrin thrombi within the vessel lumens of the dermis, red blood cell extravasation, and perivascular lymphocytic infiltrate, which are crucial for distinguishing it from other vasculopathies and vasculitides. 2
The treatment of LV is complex and often requires a multidisciplinary approach involving dermatologists, rheumatologists, and hematologists. Therapeutic strategies aim to address both the thrombotic and inflammatory aspects of the disease. While no standardized treatment protocol exists, a combination of anticoagulants like warfarin and vasodilators such as pentoxifylline and nifedipine has shown promise in managing symptoms, inducing remission, and preventing the recurrence of ulcerations. 3
Case Report
A 31-year-old female patient sought consultation in our Rheumatology Unit due to a persisting concern of recurring painful ulcerations predominantly affecting both her legs and feet, persisting over a period of 4 years. She had no history suggestive of any relevant medical or familial predispositions toward vascular or autoimmune diseases. Upon thorough review of her clinical profile, no indications of associated symptoms such as arthralgia or dermatological manifestations were reported. Her medication history was unremarkable, and she negated any prior or ongoing use of tobacco or illicit substances.
A detailed physical examination was conducted, which revealed a distinct reticular pattern reminiscent of livedo reticularis distributed across her body (Figure 1). Furthermore, the examination revealed multiple chronic unhealed ulcers, specifically localized to the lateral malleolus and the dorsum of the left foot, in addition to white scarring in the lower legs and ankles (Figure 2). Preliminary diagnostic considerations spanned across a broad spectrum of conditions, including thrombophilia, hematologic malignancies, immune-mediated vasculitis, connective tissue diseases of autoimmune nature, arterial diseases, vascular proliferations, diabetes, and medication-induced leg ulcers.
Figure 1.
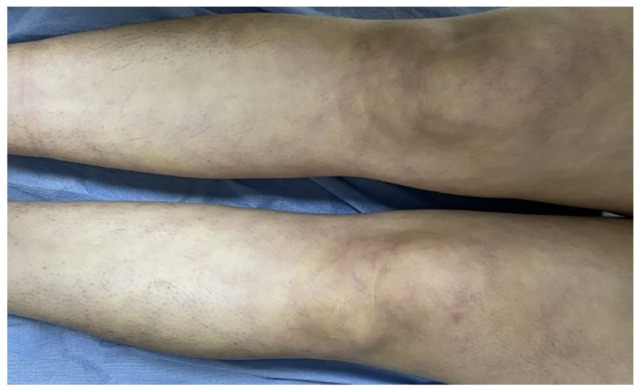
Livedo reticularis all over her lower limbs.
Figure 2.
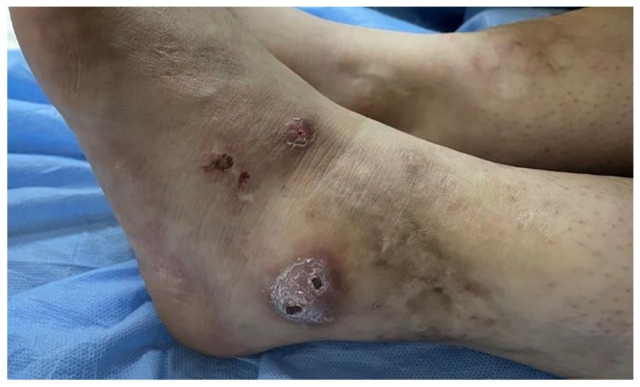
Unhealed ulcers over the lateral malleolus.
Subsequently, the patient was referred for a dermatology consultation, where a lesional skin biopsy was performed. The histopathological analysis of the biopsy sample showed fibrin thrombi within vessel lumens, extravasation of red blood cells, and perivascular lymphocyte infiltration (Figure 3). These findings were consistent with a diagnosis of LV.
Figure 3.
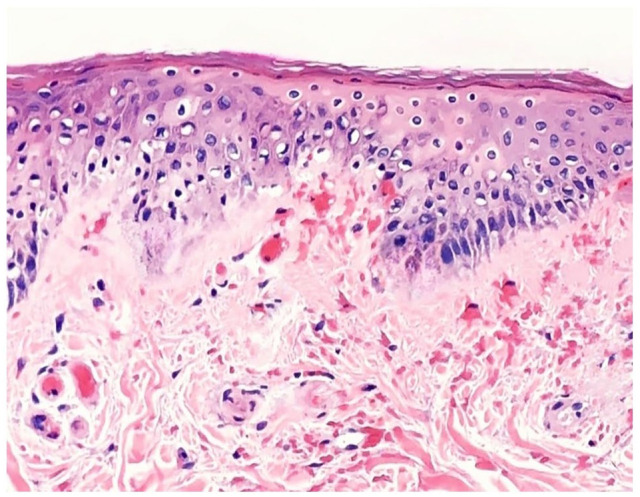
Skin biopsy histopathology analysis showing fibrin thrombi within vessel lumens, extravasation of red blood cells, and perivascular lymphocyte infiltration.
To confirm this diagnosis, the patient underwent an exhaustive series of tests to rule out hypercoagulable and autoimmune disorders. Extensive investigations for underlying diseases associated with similar vascular abnormalities were performed (Table 1) and were negative. Moreover, we performed a comprehensive imaging suite that included Doppler ultrasound of both lower limbs, neck ultrasound, abdominal ultrasound, and chest radiography. Neither the laboratory investigations nor the imaging studies revealed any abnormalities that could account for the patient’s clinical condition.
Table 1.
Extensive Blood Tests Screen.
| Test | Result | Normal ranges |
|---|---|---|
| Hemoglobin | 13 | 12-14.5 g/dL |
| WBC | 4500 | 4000-11 000 103/μL |
| PLT | 356 | 150-450 103/μL |
| Glucose | 80 | 70-100 mg/dL |
| Serum creatinine | 0.4 | 0.6-1.3 mg/dL |
| Blood urea | 11 | 10-40 g/dL |
| ALT | 24 | 7-56 IU/L |
| AST | 12 | 10-40 IU/L |
| ALP | 119 | 44-147 IU/L |
| Total bilirubin | 0.3 | 0.1-1.2 mg/dL |
| Serology for hepatitis B/C and HIV | Negative | Negative |
| Erythrocyte sedimentation rate | 12 | Women: <20 mm/h |
| C-reactive protein | 1 | <5.0 mg/L |
| Rheumatoid factor | 14 | <30 IU |
| Antinuclear antibody | 0.4 | <1.1 IU |
| Anti-double-stranded DNA antibody | 0.2 | < 1.1 IU |
| Extractable nuclear antigen antibodies | Negative | Negative |
| Lupus anticoagulants | 39 | 30-45 seconds |
| Anticardiolipin antibody | 9 | <18 IU/mL |
| Beta-2 glycoprotein antibody | 7 | <18 IU/mL |
| C-ANCA | 10 | <18 IU/mL |
| P-ANCA | 6 | <18 IU/mL |
| C3 | 111 | 90-180 mg/dL |
| C4 | 26 | 10-40 mg/dL |
| Cryoglobulins | Negative | Negative |
| Cryofibrinogens | Negative | Negative |
| Protein C levels | 130 | 70%-140% of normal |
| Protein S levels | 77 | 60%-130% of normal |
| Antithrombin III | 91 | 80%-120% of normal |
| Prothrombin mutation | Negative | Negative |
| Factor V Leiden mutation | Negative | Negative |
| Homocysteine level | 6 | 4-15 mol/L |
| Serum protein electrophoresis | Normal | Normal protein distribution |
Upon correlating the clinical presentation with the histopathological findings, in conjunction with the negative results from the extensive laboratory and imaging workup, we reached a diagnosis of primary LV. Subsequent to this diagnosis, the patient was initiated on a therapeutic regimen comprising pentoxifylline, nifedipine, and warfarin. This comprehensive treatment approach resulted in a notable improvement in her condition, as evidenced by the complete resolution of the pre-existing skin ulcers (Figure 4).
Figure 4.
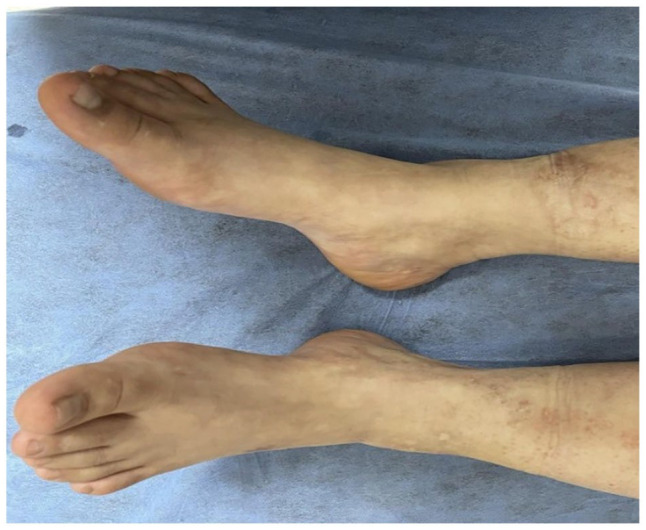
Complete healing of the ulcer following treatment.
She remained free of any new ulceration for a substantial duration of over 5 months, as recorded during her routine follow-up visits at our clinic; the medications were tapered off and the patient was told to return if any of the symptoms reappeared, but the patient was free of these symptoms until this day.
Discussion
The case of primary LV presented herein illustrates the complexities involved in diagnosing and managing this rare thrombotic skin disorder. Livedoid vasculopathy predominantly affects young to middle-aged adults, with a higher prevalence in females. The clinical hallmark of LV includes painful ulcerations, typically located on the lower extremities, which heal slowly and often result in atrophic white scars known as “atrophie blanche.” The recurrent nature of these ulcers significantly impairs patients’ quality of life, emphasizing the need for effective diagnostic and therapeutic strategies. 4
Histopathological examination is crucial for confirming LV, as it reveals fibrin thrombi within the dermal blood vessels, red blood cell extravasation, and perivascular lymphocytic infiltrates. These findings differentiate LV from other vasculopathies and vasculitides, which may present with similar clinical features but have different underlying mechanisms and treatment approaches. In this case, the patient’s biopsy results were consistent with LV, supporting the clinical diagnosis. 5
The extensive laboratory workup performed in this case, including tests for thrombophilia, autoimmune disorders, and other systemic conditions, is essential to exclude secondary causes of LV. Conditions such as antiphospholipid syndrome, systemic lupus erythematosus, and protein C or S deficiencies can present with similar clinical features and must be ruled out to confirm a diagnosis of primary LV. The negative results from these investigations in our patient helped establish the diagnosis of primary LV, guiding the subsequent management plan. 6
Treatment of LV remains challenging due to its multifactorial etiology and the lack of standardized therapeutic protocols. The primary goals of treatment are to prevent new ulcerations, promote healing of existing ulcers, and alleviate pain. Anticoagulants, such as warfarin, are commonly used to address the thrombotic component of the disease by preventing further thrombus formation. In this case, the patient was treated with warfarin, which contributed to the resolution of her ulcers. 7
Vasodilators like pentoxifylline and nifedipine are also integral to the management of LV. Pentoxifylline improves microcirculation and reduces blood viscosity, while nifedipine, a calcium channel blocker, enhances blood flow by dilating peripheral blood vessels. The combination of these medications in our patient’s treatment regimen led to significant clinical improvement, highlighting their efficacy in managing LV. 8
The multidisciplinary approach in this case, involving dermatologists, rheumatologists, and hematologists, underscores the importance of comprehensive care in LV management. This collaborative effort is crucial for accurately diagnosing the condition, excluding differential diagnoses, and implementing an effective treatment plan tailored to the patient’s needs. 9
Despite the successful outcome in this case, it is important to recognize that LV is a chronic condition with potential for recurrence. Long-term follow-up is essential to monitor for new ulcerations and to adjust treatment as needed. Educating patients about the chronic nature of the disease, the importance of medication adherence, and strategies to minimize ulcer risk is vital for maintaining remission and improving quality of life. 10
Conclusions
Livedoid vasculopathy has a wide set of differentials, including thrombophilia, malignancies, autoimmune diseases, and peripheral arterial disease. In cases of primary disease like this one, a combination of pentoxifylline, nifedipine, and warfarin was required to achieve remission and prevent skin ulceration. A multidisciplinary team including a dermatologist, hematologist, and rheumatologist is required in cases of primary LV to exclude a wide set of differentials and reach the correct diagnosis. Livedoid vasculopathy has a wide set of differentials, including thrombophilia, malignancies, autoimmune diseases, and peripheral arterial disease. In cases of primary disease like this one, a combination of pentoxifylline, nifedipine, and warfarin was required to achieve remission and prevent skin ulceration. A multidisciplinary team including a dermatologist, hematologist, and rheumatologist is required in cases of primary LV to exclude a wide set of differentials and reach the correct diagnosis.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Ahmed Qasim Mohammed Alhatemi  https://orcid.org/0009-0004-9076-8220
https://orcid.org/0009-0004-9076-8220
References
- 1. Kang S, Amagai M, Bruckner AL, et al. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019:2532-2533. [Google Scholar]
- 2. Weishaupt C, Strölin A, Kahle B, et al. Characteristics, risk factors and treatment reality in livedoid vasculopathy: a multicentre analysis. J Eur Acad Dermatol Venereol. 2019;33(9):1784-1791. [DOI] [PubMed] [Google Scholar]
- 3. Micieli R, Alavi A. Treatment for livedoid vasculopathy: a systematic review. JAMA Dermatol. 2018;154:193-202. [DOI] [PubMed] [Google Scholar]
- 4. Alavi A, Hafner J, Dutz JP, et al. Livedoid vasculopathy: an in-depth analysis using a modified Delphi approach. J Am Acad Dermatol. 2013;69(6):1033-1042. [DOI] [PubMed] [Google Scholar]
- 5. Poletti ED, Sandoval NRM, González JLM, et al. Vasculopatía livedoide: significado actual. Comunicación de dos casos. Dermatol Rev Mex. 2008;52:175-181. [Google Scholar]
- 6. Mohammed MJ, Hashim HT, Al-Obaidi AD, Al Shammari A. A novel overlap syndrome: rheumatoid arthritis, Sjogren’s syndrome, antiphospholipid syndrome, and dermatomyositis. Clin Case Rep. 2023;11(4):e7274. doi: 10.1002/ccr3.7274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hairston BR, Davis MD, Pittelkow MR, Ahmed I. Livedoid vasculopathy: further evidence for procoagulant pathogenesis. Arch Dermatol. 2006;142(11):1413-1418. [DOI] [PubMed] [Google Scholar]
- 8. Khenifer S, Thomas L, Balme B, et al. Livedoid vasculitis associated with a double heterozygous Factor V Leiden and prothrombin G20210A gene mutation. Clin Exp Dermatol. 2009;34(8):e113. [DOI] [PubMed] [Google Scholar]
- 9. Lee SS, Ang P, Tan SH. Clinical profile and treatment outcome of livedoid vasculitis: a case series. Ann Acad Med Singap. 2003;32(6):835-839. [PubMed] [Google Scholar]
- 10. Khenifer S, Thomas L, Balme B, et al. Livedoid vasculopathy: thrombotic or inflammatory disease? Clin Exp Dermatol. 2010;35:693-698. [DOI] [PubMed] [Google Scholar]


