Scheme 1. General Synthetic Routes for the Construction of Evaluated Ligands.
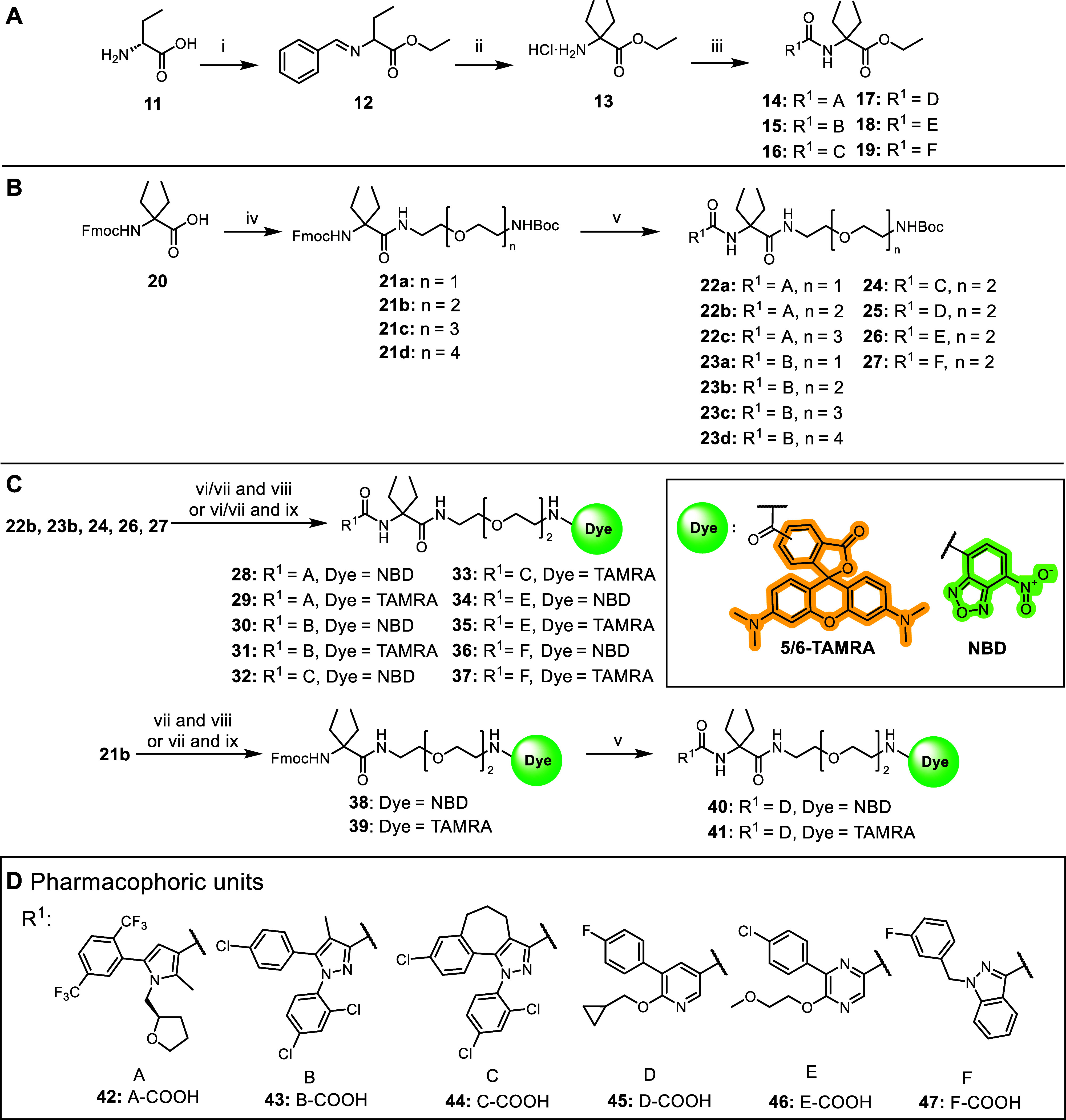
(A) Synthesis of the novel DEG ethyl ester ligands 14–19: reagents and conditions: (i) (a) SOCl2, EtOH, 0 °C to reflux; 5 h; (b) benzaldehyde, TEA, DCM, MgSO4, rt, 30 h; (ii) (a) KHDMS, EtI, THF, −70 °C to rt, 24 h; (b) HCl, Et2O, 0 °C to rt, 15 h; (iii) 42–47, HATU, DIPEA, DMF, 3 h, rt. (B) Synthesis of linker library 22a–27. Reagents and conditions: (iv) HATU, BocNH–CH2CH2(OCH2CH2)n–NH2 (n = 1–4), DIPEA, DMF, 3 h, rt; (v) DBU, DMF, then HOAt, then 42–47, HATU, DIPEA, DMF, 3 h 6 d, rt-45 °C. (C) Synthesis of fluorescent probes 28–37: reagents and conditions: for 28 and 29 (vi) HFIP, MW, 90 min, 150 °C. For 30, 32, 34, 36 (vii) TFA, DCM, 2 h, rt then (viii) NBD-F, DIPEA, DMF, 18 h. (ix) TAMRA-COOH, EDCI, HOAt, DIPEA, DMF, 20 h, rt or TAMRA-SE, DIPEA, DMF, 2 h, rt. Synthesis of fluorescent probes 40–41: reagents and conditions: (vii) TFA, DCM, 2 h, rt. (viii) NBD-F, DIPEA, DMF, 18 h. (ix) TAMRA-COOH, EDCI, HOAt, DIPEA, DMF, 20 h, rt or TAMRA-SE, DIPEA, DMF, 2 h, rt. (v) DBU, DMF, then HOAt, then 45, HATU, DIPEA, DMF, 3 h, rt. (D) Pharmacophoric carboxylic acid units 42–47 derived from 5–10. For synthesis of probes 51 and 52, see the Experimental Section.54
