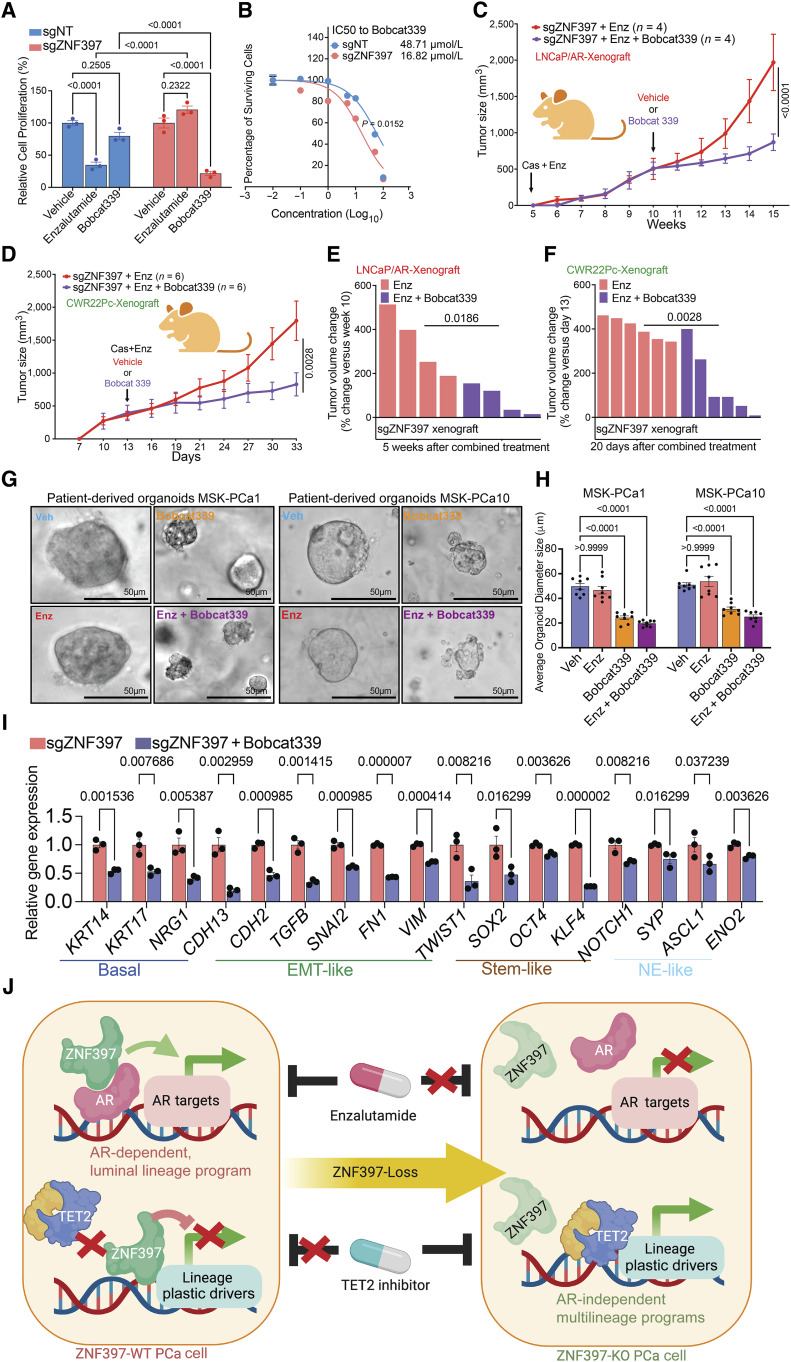
Figure 7.
Targeting TET2-driven epigenetic rewiring to overcome resistance. A, Relative cell proliferation of LNCaP/AR cells transduced with annotated guide RNAs and treated with vehicle (DMSO), enzalutamide (10 µmol/L for 7 days), or Bobcat339 (10 µmol/L for 7 days). B, Dose–response curve of ZNF397-KO and wild-type cells treated with TET2 inhibitor Bobcat339. P values were calculated by non-linear regression with extra sun-of-squares F test. C, Tumor growth curve of xenografted ZNF397-KO LNCaP/AR cells in castrated mice treated with enzalutamide. The mice were randomly separated into two groups when their tumors reached 500 mm3 and treated with vehicle or Bobcat339. D, Tumor growth curve of xenografted ZNF397-KO CWR22Pc cells in intact mice. The mice were randomly separated into two groups and castrated when their tumors reached 500 mm3 and treated with vehicle + enzalutamide or Bobcat339 + enzalutamide. E, Waterfall plot displaying changes in tumor size of xenografted ZNF397-KO LNCaP/AR cells after 5 week of combined treatments. F, Waterfall plot displaying changes in tumor size of xenografted ZNF397-KO LNCaP/AR cells after 20 days of combined treatments. For C–F, Enz denotes enzalutamide treatment at 10 mg/kg. Bobcat 339 denotes Bobcat339 treatment at 10 mg/kg. n = number of independent xenografted tumors in each group. G, Bright field pictures represent the 3D-cultured patient derived organoid models, treated with vehicle (Veh), 5 µmol/L enzalutamide (Enz), 10 µmol/L Bobcat339 or enzalutamide + Bobcat339 for 7 days. H, Bar plots represent the size of the 3D-cultured patient derived organoids, treated with vehicle (Veh), 5 µmol/L enzalutamide (Enz), 10 µmol/L Bobcat339 or enzalutamide + Bobcat339 for 7 days. I, Relative gene expression levels of canonical lineage specific marker genes in ZNF397-KO and wild-type LNCaP/AR cells treated with vehicle (DMSO) or Bobcat339 (10 µmol/L for 5 days), measured by qPCR assay. Data are normalized to vehicle-treated cells. J, A schematic figure illustrates the bifurcated role of ZNF397 as a critical coactivator of AR and a suppressor of TET2-dependent lineage plasticity. ZNF397 deficiency in prostate cancer facilitates the transition of prostate cancer cells from an AR-driven luminal lineage to a TET2-driven, multilineage, and lineage-plastic state, which no longer responded to AR-targeted therapy. A yet-to-be identified TET2 cofactor is depicted as a yellow protein. For all panels unless otherwise noted, n = 3 independently treated cell cultures and mean ± SEM are presented. P values were calculated using two-way ANOVA with Bonferroni multiple-comparison test. Schematic figure was created with BioRender.com. See also Supplementary Fig. S10.