Abstract
The innate immune system eliminates bloodstream pathogens such as Escherichia coli in part through complement protein deposition and subsequent bacterial death (i.e., “serum killing”). Some E. coli strains have developed mechanisms to resist serum killing, though the extent of variation in serum killing among bloodstream infection (BSI) isolates and the clinical impact of this variation is not well understood. To address this issue, we developed a novel assay that uses flow cytometry to perform high throughput serum bactericidal assays (SBAs) with E. coli BSI isolates (n = 183) to define the proportion of surviving bacteria after exposure to serum. We further determined whether E. coli resistance to serum killing is associated with clinical outcomes (e.g., in-hospital attributable mortality, in-hospital total mortality, septic shock) and bacterial genotype in the corresponding patients with E. coli BSI. Our novel flow cytometry-based SBA performed similarly to a traditional SBA, though with significantly decreased hands-on bench work. Among E. coli BSI isolates, the mean proportion that survived exposure to 25% serum was 0.68 (Standard deviation 0.02, range 0.57–0.93). We did not identify associations between E. coli resistance to serum killing and clinical outcomes in our adjusted models. Together, this study describes a novel flow cytometry-based approach to the bacterial SBA that allowed for high-throughput testing of E. coli BSI isolates and identified high variability in resistance to serum killing among a large set of BSI isolates.
Introduction
Escherichia coli is one of the most widely studied bacterial species [1,2]. E. coli has significant variability in its genetic repertoire, with genetic elements that promote colonization, disruption of epithelial barriers, and tissue invasion [3]. Invasion of E. coli into the bloodstream leads to bacteremia, sepsis, and a high risk of death [4–6]. Our first line of defense against E. coli in the bloodstream is the complement system, a component of innate immunity [7]. The complement system is a regulated network of proteins in the blood that is composed of three pathways: classical, lectin, and alternative. Each pathway is activated through different means, such as binding of antibody, mannose-binding lectin, or C3b to bacteria, respectively [7–11]. Activation of any pathway can result in formation of the membrane attack complex (MAC), a pore in the bacterial plasma membrane that triggers pathogen death through osmolysis [7,9,12]. Given that bacteria are killed by complement proteins within the blood (or serum), this process is referred to as serum killing [13].
While the human complement system is designed to eliminate bacteria from the bloodstream, some E. coli strains have evolved mechanisms to resist killing (i.e., serum resistance) [13,14]. For example, the presence of capsular polysaccharides may prevent MAC binding and secretion of proteases may degrade complement proteins [13–16]. However, the degree to which clinical strains of E. coli are resistant to serum killing, and the impact of this resistance on patient outcomes, is unknown. One of the challenges in addressing this issue is the extensive time and labor needed to perform traditional serum bactericidal assays (SBAs) with large collections of bacterial isolates [17]. To address this challenge, we describe in this report the development and validation of a flow cytometry-based high throughput SBA. We used this novel approach to define the variability in resistance to serum killing within a large set of E. coli BSI isolates, and then determined the extent to which this resistance is associated with patient clinical outcomes (e.g., septic shock, total in-hospital mortality, attributable in-hospital mortality) and bacterial genotype.
Methods
Study population and definitions
The patient clinical data and bacterial isolates were obtained from the Duke Blood Stream Infection Biorepository (BSIB). The BSIB contains prospectively collected clinical data and bacterial BSI isolate from >4000 unique adult inpatients at Duke University Health System with monomicrobial Gram-negative bacterial BSI since 2002. The patients in this study were enrolled between January 1, 2002 and December 31, 2015. The clinical data was accessed on January 5, 2023. Written informed consent was obtained from all study participants or their legal representatives, and the study was approved by the Duke University Institutional Review Board. Hospital-acquired infection was defined as infection beginning ≥48 hours after hospital admission [18]. Community-acquired bloodstream infection was defined as an infection beginning <48 hours after hospital admission. Community-acquired bloodstream infection was further subdivided into the following: 1) community-acquired, healthcare-associated bloodstream infection, and 2) community-acquired, non-healthcare-associated bloodstream infection. The community-acquired, healthcare-associated bloodstream infection definition was modified from Friedman et al. [18], and defined as a bloodstream infection beginning prior to 48 hours after hospital admission in patients that meet one or more of the following criteria: hospitalized in the past 90 days, resident of a nursing home or long-term care facility, actively receiving home intravenous therapy, received wound care or specialized nursing care in previous 30 days, received hemodialysis in past 30 days, immunosuppressed (e.g., presence of metastatic cancer, history of a solid organ or hematological transplant, chemotherapy in last 30 days, currently on immunosuppressive medication for any reason), or surgery in last 180 days. Community-acquired, non-healthcare-associated bloodstream infection is any community-acquired bloodstream infection not meeting the criteria for healthcare-associated bloodstream infection. The source of infection refers to the primary focus of the bloodstream infection (e.g., urine/pyelonephritis, line, etc.). Acute Physiology and Chronic Health Evaluation II (APACHE-II) score [19] was calculated at the time of initial positive blood cultures. In-hospital mortality was defined as death prior to hospital discharge. Recurrent bloodstream infection was defined as present if there was a clinical and microbiological resolution of the initial episode of infection after treatment, but culture-confirmed E. coli (same antibiogram) was documented within the index hospital admission. Complications of E. coli bloodstream infection including septic shock, acute kidney injury (AKI), acute lung injury / acute respiratory distress syndrome (ALI/ARDS), and disseminated intravascular coagulation (DIC) were defined per standard guidelines [20–23]. Appropriate antibiotic therapy is defined as the receipt of an antibiotic to which the bacteria is susceptible. Appropriate antibiotic therapy was determined daily from the date of the index positive blood culture (day 0) to hospital discharge or death. Antimicrobial susceptibility testing was performed by the Duke Clinical Microbiology Lab using standard techniques. The multidrug resistant (MDR) phenotype was defined as in Magiorakos et al. [24].
Traditional serum bactericidal assay (SBA)
As in the standard approach for performing an SBA, E. coli isolates were streaked onto LB plates and incubated at 37˚C overnight (ON). One milliliter LB cultures were inoculated from single colonies and incubated at 37˚C ON while shaking at 225 rpm. The cultures were spun down at 13,000xg for 3 minutes and the cells washed twice with PBS. The cultures were diluted with PBS to OD600 of 0.10. The serum (0%, 25%, 50%) was prepared using pooled human serum (Sigma-Aldrich, catalog number H4522) and PBS. Then, 180 μL of serum (0%, 25%, or 50%) and 20 μL of the bacterial isolate were mixed by pipetting in a 96-well plate. Plates were incubated at 37˚C for 30 minutes and centrifuged at 3,000 rpm for 20 minutes. Cells were washed twice with PBS and serially diluted 1000-fold with PBS. Fifty microliters of the diluted samples were plated on LB and incubated ON at 37˚C. Colony forming units (CFU) were counted and recorded. The proportion of living colonies for both 25% serum and 50% serum samples were calculated using the formulas below:
Novel flow cytometry-based SBA
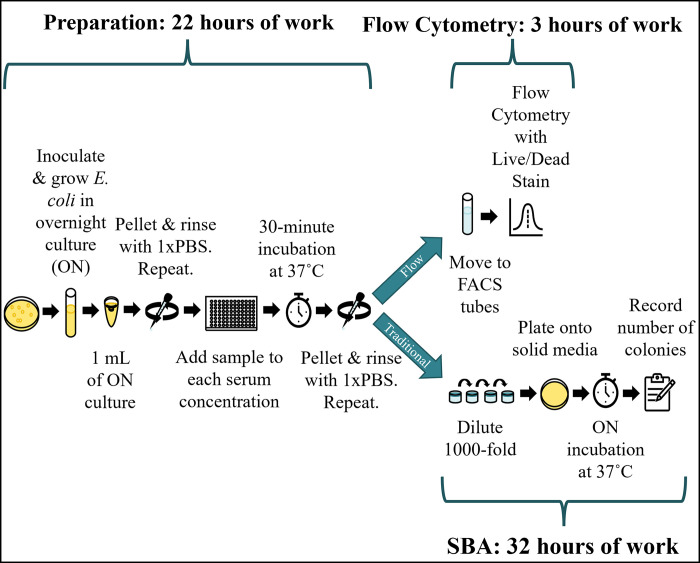
The initial steps of our novel flow cytometry-based SBA, including culture growth, washes, dilution, incubation with serum, and subsequent washes with PBS were identical to the traditional SBA protocol described above. In this new approach, the bacteria were then resuspended in PBS and transferred to FACS tubes. The live/dead stain 7-Aminoactinomycin D (7-AAD; Invitrogen, catalog number A1310) was added at a final concentration of 40 μg/mL. The samples were analyzed with a BD Biosciences FACS Canto machine. In total, 10,000 events were recorded using an excitation wavelength of 488 nm and a detection wavelength of 647 nm. The recorded values were used to produce the proportion of living cells after exposure to 25% or 50% serum using the formulas described above. For assay validation, four biological replicates across two experiments were performed. Given the low variance noted in the validation assay, serum susceptibility assays were performed in duplicate on the remaining BSI isolates. An overall schematic of the traditional and flow cytometry-based SBA approaches is shown in Fig 1.
Fig 1. Workflow and duration differences between traditional serum bactericidal assay (SBA) and flow cytometry-based SBA methods.
Both the traditional and flow-based methods begin the same way. After E. coli strains are incubated with serum and washed with PBS, the two approaches diverge (blue arrows). The blue bars indicate the approximate duration for a single person to perform an experiment with a full 96-well plate of samples. This corresponds to 16 E. coli strains exposed to 0%, 25%, and 50% serum, performed in duplicate. Abbreviations: FACS, fluorescent activated cell sorting; ON, overnight; PBS, phosphate-buffered saline.
Statistical analyses
In the description of patient and bacterial characteristics, continuous variables were reported as means with standard deviations. Dichotomous variables were reported as counts and percentages. T-tests were used to examine associations between E. coli resistance to serum killing and clinical outcomes or bacterial sequence type. Two-way analysis of variance (ANOVA) tests were used to validate our novel flow cytometry-based method relative to the traditional SBA. This test accounted for both the bacterial isolate (high, medium, and low serum susceptibility isolates were tested) and the method for determining serum susceptibility (flow cytometry-based versus traditional SBA). Chi-square tests were used to broadly identify differences in serum susceptibility among E. coli phylogroups and sequence types, and t-tests were used to identify differences in susceptibility between particular genetic groups (e.g., ST131) and all others. For the serum susceptibility histogram, a Gaussian line of best fit was generated using a nonlinear regression approach (GraphPad Prism, Boston, MA). In the adjusted analyses, logistic regression models were generated to determine associations. Adjusted models were generated for clinical outcomes including total in-hospital mortality, attributable in-hospital mortality (i.e., death due to infection as opposed to other causes), and septic shock. Model covariates included age, gender, race, route of infection (e.g., hospital-acquired infection, etc.), source of infection, hematopoietic or solid organ transplant, diabetes mellitus, recent corticosteroid use (within 30 days prior to BSI), HIV, recent surgery (within 30 days prior to BSI), days to effective antibiotics, chronic health APACHE-II score, and E. coli resistance to serum killing. E. coli resistance to serum killing for each BSI isolated was represented as the proportion of live bacteria after exposure to 25% serum relative to the 0% serum control. Therefore, this variable could range from 0 (fully killed by 25% serum) to 1 (fully resistant to serum killing). The chronic health portion of the APACHE-II describes whether a patient has severe organ system insufficiency on hospital admission. These covariates were selected to broadly encompass the clinical factors known or thought to influence BSI outcome. Model covariates with near significant p-values (p≤0.15) in a univariable analysis were included in the final multivariable logistic regression models. P-values less than 0.05 were considered significant.
The traditional SBA (i.e., non-flow cytometry-based SBA) produced considerable variation between replicates. Therefore, data analysis was performed both with and without formal outlier removal. The data presented here involved outlier removal from the traditional SBA samples, though the overall results and findings did not differ whether outliers were removed or not. The interquartile range (IQR) technique was used for outlier removal [25]. No outlier removal was necessary for the flow cytometry-based SBA given homogeneity of results across replicates.
Whole genome sequencing and assembly
The E. coli genomic sequencing data used in this study was similarly used in a prior study [26]. DNA isolation, library construction, and assembly was described in this prior study. In brief, multilocus sequence typing (MLST) was performed using LOCUST [27]. MLST was performed by using the whole genome sequence data for each E. coli BSI isolate to determine the adk, fumC, gyrB, icd, mdh, purA, and recA alleles present in each isolate. The pattern of alleles in each isolate was then matched to the particular sequence type (ST) using a standard database [28]. Pan-genome analysis was performed through the JCVI pan-genome pipeline using the Pan-genome Ortholog Clustering Tool (PanOCT) [29,30]. E. coli phylogroups were identified through either known associations with ST (e.g., ST131 and phylogroup B2) or through the In Silico Clermont Phylotyper [31]. All genomes used in this study are available at NCBI under BioProject number PRJNA290784.
Results
Creation and validation of high throughput serum susceptibility assay
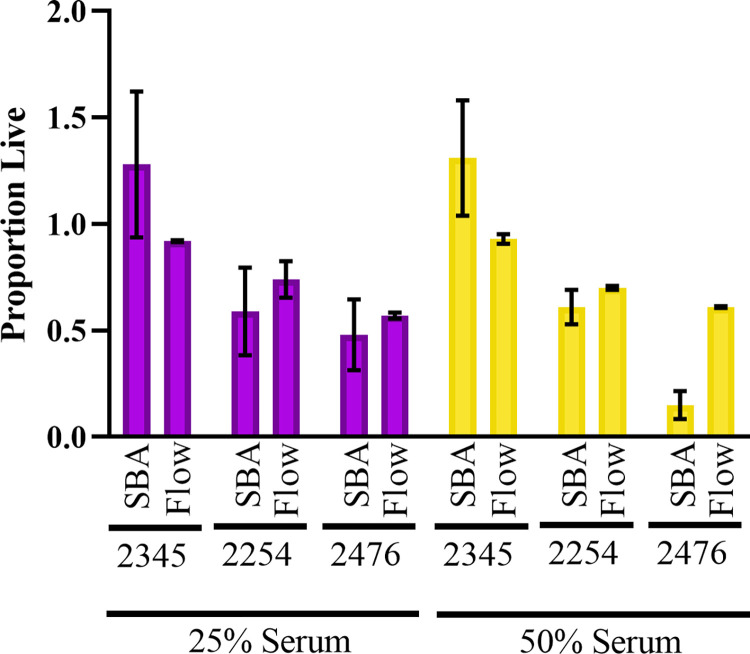
While the usual method for determining serum susceptibility is through SBAs, this method is unwieldy when used for a large number of isolates due to the need for diluting, plating, and counting colonies for each experimental sample [32,33]. Therefore, we adapted a protocol from Khan et. al. to develop a high-throughput flow cytometry-based technique to measure serum susceptibility in E. coli [34–36]. The overall schematic of time needed to complete the traditional and flow-cytometry based SBAs is shown in Fig 1, and further detailed in S1 Fig. The novel flow cytometry-based SBA decreased overall experiment duration (25 versus 54 hours) and hands-on benchwork time (8 versus 20 hours) per 16 E. coli BSI isolates (i.e., one 96-well plate in the flow cytometry-based SBA). The novel flow cytometry-based SBA successfully identified trends in serum susceptibility relative to the “gold standard” traditional SBA (Fig 2). Specifically, E. coli strains 2345, 2254, and 2476 exhibited high, medium, and low resistance to serum killing by the traditional SBA, respectively, which was similarly ordered by the novel flow cytometry-based SBA. There were no statistically significant differences in serum susceptibility between the two methodologies when isolates were exposed to either 25% serum (p = 0.76) or 50% serum (p = 0.79).
Fig 2. Validation of flow-cytometry-based serum bactericidal assay (SBA).
The serum susceptibility of E. coli strains 2345, 2254, and 2476 was determined through both a traditional SBA (SBA) and our novel flow cytometry-based (Flow) approach. The number of live and dead bacteria was determined in the presence of 0%, 25%, and 50% serum. In each case, the proportion of surviving bacteria (i.e., the number of live bacteria in serum relative to the serum-free control) is plotted. Relative differences in serum susceptibility between the E. coli strains (e.g., low, medium, and high susceptibility) were the same with the two experimental approaches (25% serum: p = 0.76; 50% serum: p = 0.79). The traditional SBA experiment included five biological replicates across two experiments. The flow cytometry-based assay included four biological replicates across two experiments.
Resistance to serum killing among E. coli BSI isolates
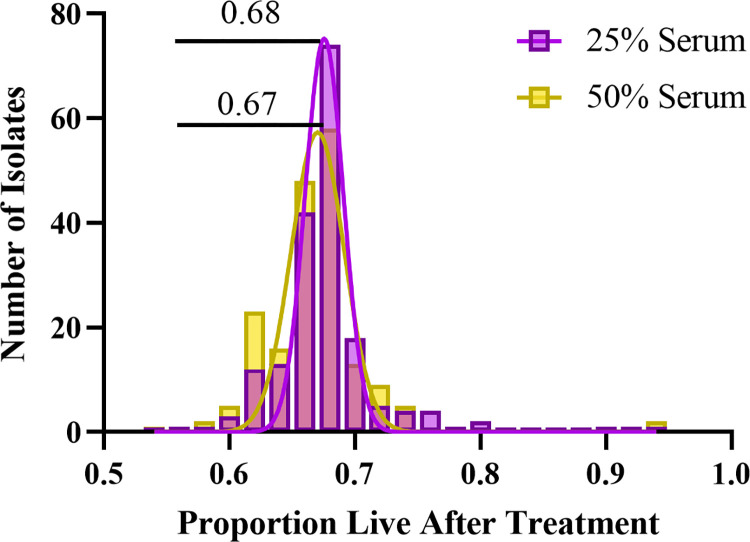
In total, 183 patients with E. coli BSI were included in this study. To investigate the serum susceptibility of these isolates, we used our novel flow-based SBA approach to determine the proportion of surviving bacteria after exposure to serum (Fig 3). The mean proportion that survived after exposure to 25% serum, relative to 0% serum, was 0.68 (standard deviation 0.02). The mean proportion that survived after exposure to 50% serum, relative to 0% serum, was 0.67 (standard deviation 0.02). While most of the E. coli BSI isolates demonstrated serum survival within a relatively narrow range (0.60–0.75 proportion survival), there were outliers that were both more resistant to serum (n = 10 isolates had >0.75 survival after exposure to 25% serum) and less resistant to serum (n = 2 isolates had <0.60 survival after exposure to 25% serum).
Fig 3. Distribution of susceptibility to serum killing among E. coli bloodstream infection (BSI) isolates.
E. coli BSI isolates (n = 183) were treated with either 25% serum (purple) or 50% serum (yellow), and the number of surviving bacteria relative to a 0% serum control was determined. The distributions of the proportion of surviving bacteria are shown.
Association between E. coli resistance to serum killing and patient clinical outcomes
The clinical characteristics and outcomes of the 183 patients with E. coli bloodstream infection are shown in Table 1. The patients had a mean age of 63 years (SD 15 years), and 87 (48%) were female. The most common source of BSI was the urinary tract (84/183 [46%]). There was significant antibiotic resistance as 123 (67%) were multidrug resistant. Overall outcomes were poor with 20% total in-hospital mortality (37/183), 13% attributable in-hospital mortality (24/183), and 25% of patients experiencing septic shock (45/183). We did not identify any significant associations between serum susceptibility and the patient and bacterial variables in Table 1.
Table 1. Characteristics and outcomes of patients with E. coli bloodstream infections (BSI).
Abbreviations: HIV, human immunodeficiency virus; SD, standard deviation.
| Patient characteristics | n (%) N = 183 |
|---|---|
| Age in years (mean [SD]) | 63 (15) |
| Female | 87 (48) |
| Race | |
| White | 119 (65) |
| Black | 48 (26) |
| Other | 16 (9) |
| Medical comorbidities | |
| Transplant (solid organ or hematopoietic stem cell) | 22 (12) |
| Diabetes mellitus | 65 (36) |
| Surgery past 30 days | 47 (26) |
| Hemodialysis | 18 (10) |
| Corticosteroid use in past 30 days | 40 (27) |
| HIV | 2 (1) |
| Acute APACHE II score (mean [SD]) | 8 (6) |
| Chronic APACHE II score (mean [SD]) | 4 (2) |
| Source of BSI | |
| Urine/pyelonephritis | 84 (46) |
| Biliary tract | 15 (8) |
| Line | 5 (3) |
| Abscess | 6 (3) |
| Pneumonia | 8 (4) |
| Skin / soft tissue | 10 (5) |
| Other | 23 (13) |
| None identified | 32 (17) |
| Route of infection | |
| Hospital-acquired | 35 (19) |
| Community acquired/Healthcare-associated | 116 (63) |
| Community-acquired/Non-healthcare-associated | 32 (17) |
| Days to appropriate antibiotics | |
| 0 days | 111 (61) |
| 1 day | 38 (21) |
| 2 days | 17 (9) |
| ≥3 days | 15 (8) |
| Unknown | 2 (1) |
| Patient outcomes |
n (%)
N = 183 |
| Total in-hospital mortality | 37 (20) |
| Attributable in-hospital mortality | 24 (13) |
| Complications of BSI | |
| Septic shock | 45 (25) |
| Acute kidney injury | 60 (33) |
| Acute lung injury / acute respiratory distress | 5 (3) |
| Bacterial characteristics |
n (%)
N = 183 |
| Fluoroquinolone resistant | 89 (49) |
| Multidrug resistant (MDR) | 123 (67) |
| Extensively drug resistant (XDR) | 0 (0) |
We sought to determine if E. coli resistance to serum killing was associated with the clinical outcomes of total in-hospital mortality, attributable in-hospital mortality, or septic shock. We hypothesized that increased survival in 25% serum would be associated with increased rates of these patient complications. In unadjusted analyses (i.e., t-tests), however, the only association we identified was decreased attributable mortality with increased resistance to serum killing (Survival 0.68 in those that survived or died of other causes versus 0.67 in those that died due to BSI; p = 0.04). No associations between E. coli resistance to serum killing (25% serum) and total mortality (Serum survival 0.68 in those that survived to discharge versus 0.67 in those that died prior to discharge; p = 0.27) or septic shock (Serum survival 0.68 in those that did not develop septic shock versus 0.67 in those that did; p = 0.16) were identified.
Adjusted models of total in-hospital mortality and attributable in-hospital mortality are shown in S1 and S2 Tables, respectively. Clinical factors associated with increased total mortality in this cohort included increasing age (Odds ratio 1.04; 95% confidence interval 1.01–1.08; p = 0.02), community-acquired/healthcare-associated infection (relative to hospital-acquired infection) (Odds ratio 0.36; 95% confidence interval 0.13–0.99; p = 0.05), hemodialysis dependence (Odds ratio 5.43; 95% confidence interval 1.61–18.92; p<0.01), and an unknown source of BSI (Odds ratio 4.65;83 95% confidence interval 1.53–14.; p<0.01). Given that these multivariable logistic regression models involved only covariates that were significant or near significant in univariable analyses (i.e., p<0.15), E. coli resistance to serum killing was only included in the attributable mortality model; however, it was not significant (OR < 0.01, 95% CI <0.01–86.19, p = 0.26). An adjusted model of septic shock was not generated as no covariates demonstrated significance in univariable analyses.
Associations between E. coli resistance to serum killing and bacterial genetics
The phylogenetic relationships among the E. coli BSI isolates included in this study, as well as the associated resistance to serum killing and clinical outcomes metadata for each BSI isolate, is shown in S2 Fig. E. coli phylogroups included A (n = 3 [2%]), B1 (n = 7 [4%]), B2 (n = 129 [70%]), C (n = 1 [1%]), D (n = 37 [20%]), and F (n = 6 [3%]). Overall, there was no difference in serum killing among phylogroups (25% serum: p = 0.27; 50% serum: p = 0.59). Similarly, pairwise comparisons (e.g., phylogroup B2 vs. all others) did not identify phylogroups associated with higher or lower serum killing.
In total, 36 E. coli STs were identified among the BSI isolates in this study. The E. coli STs with ≥5 representatives in our study population, along with the mean and range of surviving bacteria after serum exposure, are shown in Table 2. Interestingly, the only ST that statistically differed in its resistance to serum killing was ST131, which had slightly lower resistance to serum killing relative to non-ST131 strains (ST131: mean proportion surviving in 25% serum 0.67 [SD 0.026]; non-ST131: mean proportion surviving in 25% serum 0.68 [SD 0.061]; p = 0.05).
Table 2. Resistance to serum killing among E. coli bloodstream infection isolates, stratified by multilocus sequence type (ST).
A serum bactericidal assay was performed on each E. coli isolate, and survival was determined by number of colonies in the serum-treated sample (either 25% or 50% serum) divided by the number of colonies in the negative serum control. The mean survival and range of survival values for each E. coli ST with ≥5 isolates in our study population are shown here. P-values were calculated with t-tests (e.g., proportion of surviving bacteria in ST12 relative to non-ST12 isolates). P-values ≤0.05 are in bold.
| 25% serum | 50% serum | ||||||
|---|---|---|---|---|---|---|---|
| ST | # isolates | Mean proportion surviving | Range of proportion surviving | P-value | Mean proportion surviving | Range of proportion surviving | P-value |
| 12 | 7 | 0.72 | 0.65–0.90 | 0.21 | 0.71 | 0.66–0.89 | 0.20 |
| 69 | 12 | 0.70 | 0.65–0.93 | 0.41 | 0.69 | 0.61–0.94 | 0.41 |
| 73 | 12 | 0.68 | 0.61–0.77 | 0.97 | 0.67 | 0.58–0.72 | 0.88 |
| 95 | 18 | 0.68 | 0.62–0.92 | 0.84 | 0.68 | 0.59–0.93 | 0.60 |
| 127 | 5 | 0.68 | 0.66–0.70 | 1.00 | 0.67 | 0.64–0.71 | 1.00 |
| 131 | 67 | 0.67 | 0.61–0.74 | 0.05 | 0.66 | 0.60–0.73 | 0.05 |
| 393 | 10 | 0.66 | 0.57–0.76 | 0.17 | 0.66 | 0.60–0.74 | 0.41 |
| 405 | 6 | 0.67 | 0.64–0.70 | 0.29 | 0.66 | 0.62–0.68 | 0.50 |
Discussion
The variability in serum susceptibility among clinical bacterial strains and its impact on the outcomes of patients is an underexplored area of research. This is an important gap as complement-mediated serum killing is the host’s first line of defense against pathogens that invade the bloodstream, and deficiencies in the complement system are associated with recurrent and severe infections [7,9,10,12,37,38]. While the mechanistic basis of the complement system has been well-studied, less is known about bacterial variability in susceptibility to complement-mediated serum killing and how susceptibility impacts the clinical outcomes of patients with bacterial infections. The lengthy and tedious nature of the current methodologies (i.e., SBAs) for performing high-throughput analyses of bacterial serum susceptibility complicates our ability to address these questions [32,33,36]. We hope that the development of a high throughput method for determining bacterial serum susceptibility, as described here, could pave the way for additional large studies into this variability. This study had three major findings, which are discussed in detail below.
First, our novel flow cytometry-based approach for measuring E. coli serum susceptibility produced results similar to the gold standard study (traditional SBA) but with decreased overall duration, hands-on bench work, and variation between biological replicates. This work extends upon prior methodological work. Fluorescent stains, coupled with flow cytometry, have been used to assess the dynamics of bacterial death secondary to irradiation, isopropanol, or antibiotics [39–43]. Luminescence-based strategies to measure bacterial killing (including complement-mediated killing) have been published, though this approach is impractical for studying large numbers of bacterial strains given the requirement for introducing a luciferase gene into the bacterial strains of interest [44–46]. Prior work has also increased the throughput of SBAs through automated colony counting [47,48], or by detecting ATP release from dead/dying bacteria [49–53]. Here we demonstrate a technique that avoids the need for any plating and colony counting yet retains the ability to count individual viable bacteria. One prior study used a fluorescent live/dead stain (propidium iodide) to assay bacterial viability following exposure to serum [54], though examined only a single bacterial strain and did not scale the approach to investigate multiple isolates.
Second, we utilized the novel flow cytometry-based SBA method to demonstrate high variation in serum resistance (~50–100% live bacteria after treatment) among E. coli bloodstream infection isolates. To our knowledge, no prior studies have systematically described the serum susceptibility of a large set of clinical E. coli BSI isolates. One study assayed serum killing among a set of 20 E. coli BSI isolates [55], while another similarly assayed serum killing among 20 E. coli BSI isolates as a control group for E. coli isolated from infected orthopedic hardware [56]. Other studies examined serum susceptibility of E. coli clinical isolates from the urinary and gastrointestinal tracts [56–58]. These prior studies have also demonstrated significant variation in resistance to serum killing among clinical E. coli isolates. These studies identified E. coli strains that had higher serum sensitivity than those in this study (e.g., as low as 1% survival relative to serum-free controls), though these prior studies differed in both the serum used (75% normal human serum from single donor) and incubation time (1–3 hours).
Third, there were no significant associations between resistance to serum killing and the clinical outcomes of interest (total in-hospital mortality, attributable in-hospital mortality, septic shock) in our adjusted models. Patient outcomes depend on a complex interplay of patient, treatment, and pathogen variables, and the impact of E. coli resistance to serum killing on patient outcomes may not be particularly significant. However, it is challenging to fully assess the impact of resistance to serum killing in the absence of patient-specific serum samples which may vary in their complement levels and activity. In a rabbit model of infective endocarditis, resistance to killing in rabbit serum was shown to be an important factor in the ability of E. coli to generate a persistent infection in this organism [59].
This study has several limitations. First, all the isolates came from a single geographic area, so some STs that are less common here will not be well represented. Second, we used commercially available pooled human serum and not serum from the original patient. There could be differences in serum susceptibility to the corresponding patient’s serum versus that observed here using pooled human serum. These differences could have contributed to the lack of association between serum resistance and patient clinical outcomes. Finally, this work does not capture additional important actions of the complement system such as opsonin-guided phagocytic activity or modulation of cytokine release.
In conclusion, complement-mediated killing of bacterial pathogens is a critical component of the human innate immune defense against bloodborne bacteria, though variation in bacterial resistance to serum killing, and its impact on clinical outcomes, is an underexplored area of study. This gap in the scientific literature is in part related to the lengthy and tedious nature of the assays traditionally used to examine resistance to serum killing among bacterial strains. To address this issue, we here described the development of a novel high-throughput, flow cytometry-based SBA that can be used to probe variations in complement-mediated killing of bacterial strains. We used this novel assay to probe resistance to complement-mediated serum killing in a large set of E. coli BSI isolates. We identified variation in resistance to serum killing among these BSI isolates, though did not identify associations between this resistance and clinical outcomes of mortality and septic shock. Future work should focus on examinations of the mechanisms of resistance to complement-mediated serum killing and its impact on clinical outcomes in other settings. While we did not identify associations between resistance to serum killing and clinical outcomes in our cohort, such associations have been identified in an animal model of bloodstream infection [59].
Supporting information
The estimates are time required for a single person to perform the experiment with 16 E. coli strains (0%, 25%, and 50% serum) in duplicate. For the flow cytometry-based SBA, this corresponds to one full 96-well plate.
(TIF)
The innermost two colored range indicate the most common multilocus sequence types and phylogroup, respectively. The outer colored strips indicate the live bacteria proportions associated with exposure to 25% serum (yellow) and 50% serum (purple) as well as the corresponding patient clinical data of attributable in-hospital mortality (orange), total in-hospital mortality (gray), and septic shock (green).
(TIF)
Covariates with p<0.15 in univariable logistic regression analyses were included in a multivariable logistic regression model. The final multivariable logistic regression model is shown here. P-values ≤0.05 are in bold.
(DOCX)
Covariates with p<0.15 in univariable logistic regression analyses were included in a multivariable logistic regression model. The final multivariable logistic regression model is shown here. P-values ≤0.05 are in bold.
(DOCX)
Data Availability
There are legal and ethical restrictions on sharing patient-level data. Sharing of clinical data requires review and approval of a Data Use Agreement by the Duke Institutional Review Board. Request for access to this data can be made by contacting Vance Fowler (vance.fowler@duke.edu) and the Duke University Health System IRB office (919-668-5111).
Funding Statement
J.T.T.; National Institute of Allergy and Infectious Diseases, National Institutes of Health; K08 AI171183; https://www.niaid.nih.gov/ V.G.F.; National Institute of Allergy and Infectious Diseases, National Institutes of Health; R01 AI165671; https://www.niaid.nih.gov/ D.E.F.; Department of Health and Human Services; U19AI110819; https://www.hhs.gov/ D.E.F.; Centers for Disease control and Prevention (CDC) Epicenter Program; U54CK000603; https://www.cdc.gov/hai/epicenters/index.html. In no case did the funders play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26(4):822–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang J, Hur HG, Sadowsky MJ, Byappanahalli MN, Yan T, Ishii S. Environmental Escherichia coli: ecology and public health implications-a review. J Appl Microbiol. 2017;123(3):570–81. [DOI] [PubMed] [Google Scholar]
- 3.Holmes CL, Anderson MT, Mobley HLT, Bachman MA. Pathogenesis of Gram-Negative Bacteremia. Clin Microbiol Rev. 2021;34(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaper JB. Pathogenic Escherichia coli. Int J Med Microbiol. 2005;295(6–7):355–6. [DOI] [PubMed] [Google Scholar]
- 5.Bonten M, Johnson JR, van den Biggelaar AHJ, Georgalis L, Geurtsen J, de Palacios PI, et al. Epidemiology of Escherichia coli Bacteremia: A Systematic Literature Review. Clin Infect Dis. 2021;72(7):1211–9. [DOI] [PubMed] [Google Scholar]
- 6.MacKinnon MC, McEwen SA, Pearl DL, Lyytikainen O, Jacobsson G, Collignon P, et al. Mortality in Escherichia coli bloodstream infections: a multinational population-based cohort study. BMC Infect Dis. 2021;21(1):606. doi: 10.1186/s12879-021-06326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajic G, Degn SE, Thiel S, Andersen GR. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015;34(22):2735–57. doi: 10.15252/embj.201591881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5(10):981–6. doi: 10.1038/ni1113 [DOI] [PubMed] [Google Scholar]
- 9.Lo MW, Woodruff TM. Complement: Bridging the innate and adaptive immune systems in sterile inflammation. J Leukoc Biol. 2020;108(1):339–51. doi: 10.1002/JLB.3MIR0220-270R [DOI] [PubMed] [Google Scholar]
- 10.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44(16):3875–88. doi: 10.1016/j.molimm.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 11.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176(3):1305–10. doi: 10.4049/jimmunol.176.3.1305 [DOI] [PubMed] [Google Scholar]
- 12.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343(1):227–35. doi: 10.1007/s00441-010-1034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abreu AG, Barbosa AS. How Escherichia coli Circumvent Complement-Mediated Killing. Front Immunol. 2017;8:452. doi: 10.3389/fimmu.2017.00452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miajlovic H, Smith SG. Bacterial self-defence: how Escherichia coli evades serum killing. FEMS Microbiol Lett. 2014;354(1):1–9. [DOI] [PubMed] [Google Scholar]
- 15.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6(2):132–42. doi: 10.1038/nrmicro1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rooijakkers SH, van Strijp JA. Bacterial complement evasion. Mol Immunol. 2007;44(1–3):23–32. doi: 10.1016/j.molimm.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 17.Abd El-Aziz AM, Elgaml A, Ali YM. Bacteriophage Therapy Increases Complement-Mediated Lysis of Bacteria and Enhances Bacterial Clearance After Acute Lung Infection With Multidrug-Resistant Pseudomonas aeruginosa. J Infect Dis. 2019;219(9):1439–47. [DOI] [PubMed] [Google Scholar]
- 18.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care—associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–7. doi: 10.7326/0003-4819-137-10-200211190-00007 [DOI] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 20.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015;43(1):3–12. doi: 10.1097/CCM.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 21.Ragaller M, Richter T. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock. 2010;3(1):43–51. doi: 10.4103/0974-2700.58663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33. doi: 10.1111/j.1365-2141.2009.07600.x [DOI] [PubMed] [Google Scholar]
- 24.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 25.Vinutha HP, Poornima B, Sagar BM. Detection of Outliers Using Interquartile Range Technique from Intrusion Dataset. Information and Decision Sciences. 2018;701:511–8. [Google Scholar]
- 26.Brumwell A, Sutton G, Lantos PM, Hoffman K, Ruffin F, Brinkac L, et al. Escherichia coli ST131 Associated with Increased Mortality in Bloodstream Infections from Urinary Tract Source. J Clin Microbiol. 2023;61(7):e0019923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkac LM, Beck E, Inman J, Venepally P, Fouts DE, Sutton G. LOCUST: a custom sequence locus typer for classifying microbial isolates. Bioinformatics. 2017;33(11):1725–6. doi: 10.1093/bioinformatics/btx045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, Alikhan NF, Mohamed K, Fan Y, Agama Study G, Achtman M. The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 2020;30(1):138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inman JM, Sutton GG, Beck E, Brinkac LM, Clarke TH, Fouts DE. Large-scale comparative analysis of microbial pan-genomes using PanOCT. Bioinformatics. 2019;35(6):1049–50. doi: 10.1093/bioinformatics/bty744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fouts DE, Brinkac L, Beck E, Inman J, Sutton G. PanOCT: automated clustering of orthologs using conserved gene neighborhood for pan-genomic analysis of bacterial strains and closely related species. Nucleic Acids Res. 2012;40(22):e172. doi: 10.1093/nar/gks757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beghain J, Bridier-Nahmias A, Le Nagard H, Denamur E, Clermont O. ClermonTyping: an easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microbial Genomics. 2018;4(7). doi: 10.1099/mgen.0.000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reller LB. Interaction of cefotaxime and desacetylcefotaxime against pathogenic bacteria. Assessment with the serum bactericidal test. Diagn Microbiol Infect Dis. 1984;2(3 Suppl):55S–61S. [PubMed] [Google Scholar]
- 33.Reller LB. The serum bactericidal test. Rev Infect Dis. 1986;8(5):803–8. doi: 10.1093/clinids/8.5.803 [DOI] [PubMed] [Google Scholar]
- 34.Wang C-Y, Wang S-W, Huang W-C, Kim KS, Chang N-S, Wang Y-H, et al. Prc Contributes to Escherichia coli Evasion of Classical Complement Mediated Serum Killing. Infection and Immunity. 2012;80(10):3399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adan A, Alizada G, Kiraz Y, Baran Y, Nalbant A. Flow cytometry: basic principles and applications. Crit Rev Biotechnol. 2017;37(2):163–76. doi: 10.3109/07388551.2015.1128876 [DOI] [PubMed] [Google Scholar]
- 36.Khan MM, Pyle BH, Camper AK. Specific and rapid enumeration of viable but nonculturable and viable-culturable gram-negative bacteria by using flow cytometry. Appl Environ Microbiol. 2010;76(15):5088–96. doi: 10.1128/AEM.02932-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroder-Braunstein J, Kirschfink M. Complement deficiencies and dysregulation: Pathophysiological consequences, modern analysis, and clinical management. Mol Immunol. 2019;114:299–311. doi: 10.1016/j.molimm.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 38.Skattum L, van Deuren M, van der Poll T, Truedsson L. Complement deficiency states and associated infections. Mol Immunol. 2011;48(14):1643–55. doi: 10.1016/j.molimm.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 39.Berney M, Hammes F, Bosshard F, Weilenmann HU, Egli T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl Environ Microbiol. 2007;73(10):3283–90. doi: 10.1128/AEM.02750-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stiefel P, Schmidt-Emrich S, Maniura-Weber K, Ren Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015;15:36. doi: 10.1186/s12866-015-0376-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ou F, McGoverin C, White J, Swift S, Vanholsbeeck F. Bead-Based Flow-Cytometric Cell Counting of Live and Dead Bacteria. Methods Mol Biol. 2019;1968:123–34. doi: 10.1007/978-1-4939-9199-0_11 [DOI] [PubMed] [Google Scholar]
- 42.Suller MT, Lloyd D. Fluorescence monitoring of antibiotic-induced bacterial damage using flow cytometry. Cytometry. 1999;35(3):235–41. doi: [DOI] [PubMed] [Google Scholar]
- 43.Ou F, McGoverin C, Swift S, Vanholsbeeck F. Absolute bacterial cell enumeration using flow cytometry. J Appl Microbiol. 2017;123(2):464–77. doi: 10.1111/jam.13508 [DOI] [PubMed] [Google Scholar]
- 44.Atosuo J, Lehtinen J, Vojtek L, Lilius EM. Escherichia coli K-12 (pEGFPluxABCDEamp): a tool for analysis of bacterial killing by antibacterial agents and human complement activities on a real-time basis. Luminescence. 2013;28(5):771–9. [DOI] [PubMed] [Google Scholar]
- 45.Deryabin DG, Polyakov EG. On-line determination of serum bactericidal activity using recombinant luminescent bacteria. Bull Exp Biol Med. 2006;142(2):234–8. doi: 10.1007/s10517-006-0336-4 [DOI] [PubMed] [Google Scholar]
- 46.Nypaver CM, Thornton MM, Yin SM, Bracho DO, Nelson PW, Jones AE, et al. Dynamics of human complement-mediated killing of Klebsiella pneumoniae. Am J Respir Cell Mol Biol. 2010;43(5):585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weerts HP, Yu J, Kaminski RW, Nahm MH. A High-throughput Shigella-specific Bactericidal Assay. J Vis Exp. 2019(144). doi: 10.3791/59164 [DOI] [PubMed] [Google Scholar]
- 48.Kim HW, Kim KH, Kim J, Nahm MH. A high throughput serum bactericidal assay for antibodies to Haemophilus influenzae type b. BMC Infect Dis. 2016;16(1):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aruta MG, Carducci M, Micoli F, Necchi F, Rossi O. Increasing the High Throughput of a Luminescence-Based Serum Bactericidal Assay (L-SBA). BioTech (Basel). 2021;10(3). doi: 10.3390/biotech10030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Necchi F, Saul A, Rondini S. Development of a high-throughput method to evaluate serum bactericidal activity using bacterial ATP measurement as survival readout. PLoS One. 2017;12(2):e0172163. doi: 10.1371/journal.pone.0172163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aruta MG, De Simone D, Dale H, Chirwa E, Kadwala I, Mbewe M, et al. Development and Characterization of a Luminescence-Based High-Throughput Serum Bactericidal Assay (L-SBA) to Assess Bactericidal Activity of Human Sera against Nontyphoidal Salmonella. Methods Protoc. 2022;5(6). doi: 10.3390/mps5060100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Micoli F, Rossi O, Conti V, Launay O, Scire AS, Aruta MG, et al. Antibodies Elicited by the Shigella sonnei GMMA Vaccine in Adults Trigger Complement-Mediated Serum Bactericidal Activity: Results From a Phase 1 Dose Escalation Trial Followed by a Booster Extension. Front Immunol. 2021;12:671325. doi: 10.3389/fimmu.2021.671325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mancini F, Micoli F, Rossi O. Setup and Characterization of a High-Throughput Luminescence-Based Serum Bactericidal Assay (L-SBA) to Determine Functionality of Human Sera against Shigella flexneri. BioTech (Basel). 2022;11(3). doi: 10.3390/biotech11030029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Virta M, Lineri S, Kankaanpaa P, Karp M, Peltonen K, Nuutila J, et al. Determination of complement-mediated killing of bacteria by viability staining and bioluminescence. Appl Environ Microbiol. 1998;64(2):515–9. doi: 10.1128/AEM.64.2.515-519.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miajlovic H, Mac Aogain M, Collins CJ, Rogers TR, Smith SGJ. Characterization of Escherichia coli bloodstream isolates associated with mortality. J Med Microbiol. 2016;65(1):71–9. [DOI] [PubMed] [Google Scholar]
- 56.Cremet L, Broquet A, Jacqueline C, Chaillou C, Asehnoune K, Corvec S, et al. Innate immune evasion of Escherichia coli clinical strains from orthopedic implant infections. Eur J Clin Microbiol Infect Dis. 2016;35(6):993–9. [DOI] [PubMed] [Google Scholar]
- 57.Hughes C, Phillips R, Roberts AP. Serum resistance among Escherichia coli strains causing urinary tract infection in relation to O type and the carriage of hemolysin, colicin, and antibiotic resistance determinants. Infect Immun. 1982;35(1):270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siegfried L, Kmetova M, Janigova V, Sasinka M, Takacova V. Serum response of Escherichia coli strains causing dyspepsia and urinary tract infection: relation to alpha-hemolysin production and O type. Infect Immun. 1995;63(11):4543–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durack DT, Beeson PB. Protective role of complement in experimental Escherichia coli endocarditis. Infect Immun. 1977;16(1):213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]