Abstract
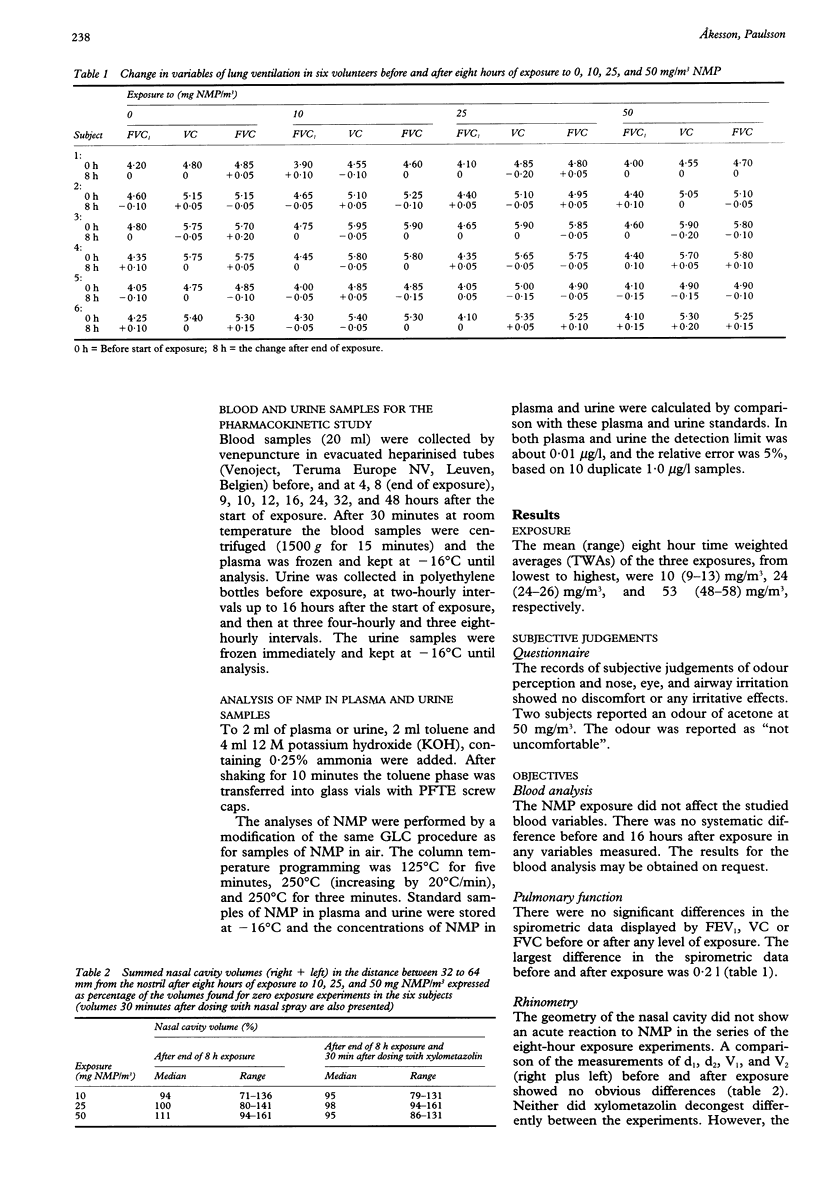
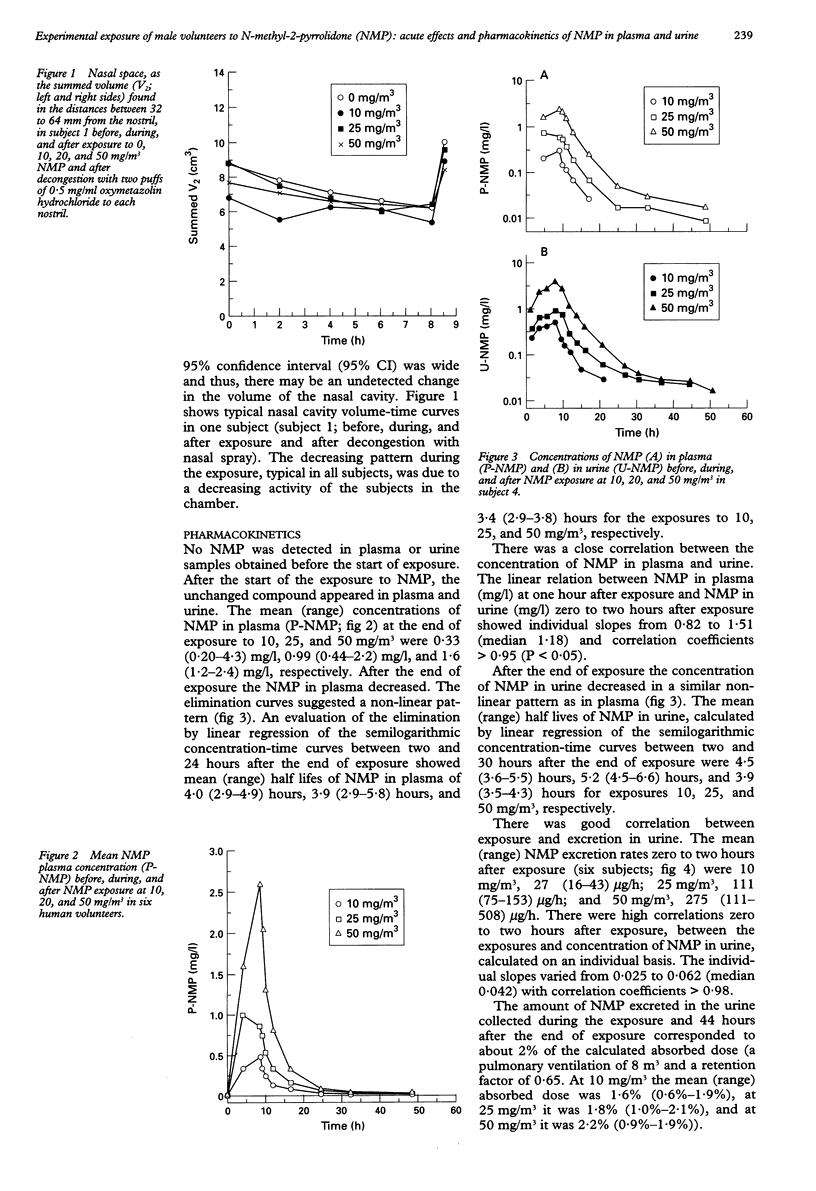
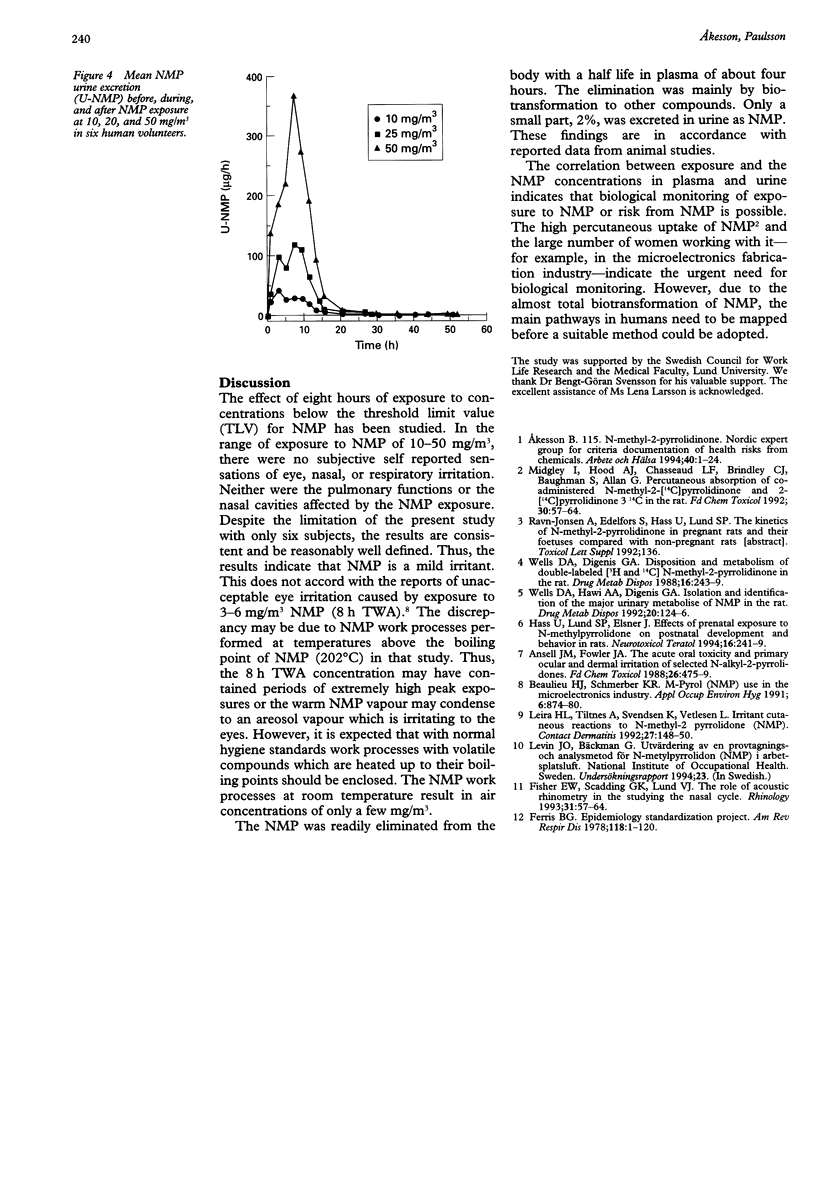
OBJECTIVES: To study the acute effects of exposure to the increasingly used solvent, N-methyl-2-pyrrolidone (NMP) in male volunteers. Further, to determine the NMP concentration in plasma and urine during and after the exposure. METHODS: Six male volunteers were exposed for eight hours on four different days to 0, 10, 25, and 50 mg/m3 NMP. Plasma was collected and urine was sampled during and after the exposure. Changes in nasal volume were measured by acoustic rhinometry and in airway resistance by spirometry. RESULTS: The eight-hour experimental exposure to 10, 25, and 50 mg/m3 did not induce discomfort to eyes or upper airways. Acute changes in nasal volume were not found, and no changes in the spirometric data could be registered. The elimination curves suggested a non-linear pattern and at the end of exposure showed mean (range) half lifes of NMP in plasma of about 4.0 (2.9-5.8) hours and in urine 4.5 (3.5-6.6) hours. The unmetabolised NMP found in urine samples collected during exposure and at the subsequent 44 hours corresponded to about 2% of the calculated absorbed dose. At the end of the exposure there was a close correlation between exposures and the plasma concentration and urinary excretion of NMP. CONCLUSIONS: NMP was absorbed through the respiratory tract and readily eliminated from the body, mainly by biotransformation to other compounds. Exposure to 10, 25, or 50 mg/m3 NMP did not cause nose, eye, or airway irritation. Thus, NMP is a mild irritant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansell J. M., Fowler J. A. The acute oral toxicity and primary ocular and dermal irritation of selected N-alkyl-2-pyrrolidones. Food Chem Toxicol. 1988 May;26(5):475–479. doi: 10.1016/0278-6915(88)90060-9. [DOI] [PubMed] [Google Scholar]
- Ferris B. G. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978 Dec;118(6 Pt 2):1–120. [PubMed] [Google Scholar]
- Fisher E. W., Scadding G. K., Lund V. J. The role of acoustic rhinometry in studying the nasal cycle. Rhinology. 1993 Jun;31(2):57–61. [PubMed] [Google Scholar]
- Hass U., Lund S. P., Elsner J. Effects of prenatal exposure to N-methylpyrrolidone on postnatal development and behavior in rats. Neurotoxicol Teratol. 1994 May-Jun;16(3):241–249. doi: 10.1016/0892-0362(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Leira H. L., Tiltnes A., Svendsen K., Vetlesen L. Irritant cutaneous reactions to N-methyl-2-pyrrolidone (NMP). Contact Dermatitis. 1992 Sep;27(3):148–150. doi: 10.1111/j.1600-0536.1992.tb05243.x. [DOI] [PubMed] [Google Scholar]
- Midgley I., Hood A. J., Chasseaud L. F., Brindley C. J., Baughman S., Allan G. Percutaneous absorption of co-administered N-methyl-2-[14C]pyrrolidinone and 2-[14C]pyrrolidinone in the rat. Food Chem Toxicol. 1992 Jan;30(1):57–64. doi: 10.1016/0278-6915(92)90137-a. [DOI] [PubMed] [Google Scholar]
- Wells D. A., Digenis G. A. Disposition and metabolism of double-labeled [3H and 14C] N-methyl-2-pyrrolidinone in the rat. Drug Metab Dispos. 1988 Mar-Apr;16(2):243–249. [PubMed] [Google Scholar]
- Wells D. A., Hawi A. A., Digenis G. A. Isolation and identification of the major urinary metabolite of N-methylpyrrolidinone in the rat. Drug Metab Dispos. 1992 Jan-Feb;20(1):124–126. [PubMed] [Google Scholar]


