Abstract
Aims
Plasma volume status (PVS), a measure of plasma volume, has been evaluated as a prognostic marker for chronic heart failure. Although the prognostic value of PVS has been reported, its significance in patients with acute decompensated heart failure (ADHF) admitted to the cardiovascular intensive care unit (CICU) remains unclear. In this study, we examined the relationship between PVS and long‐term mortality in patients with ADHF admitted to the CICU.
Methods
Between January 2018 and December 2020, 363 consecutive patients with ADHF were admitted to the Nippon Medical School Hospital CICU. Of the 363 patients, 206 (mean age, 74.9 ± 12.9 years; men, 64.6%) were enrolled in this study. Patients who received red blood cell transfusions, underwent dialysis, were discharged from the CICU or died in the hospital were excluded from the study. We measured the PVS of the patients at admission, transfer to the general ward (GW) and discharge using the Kaplan–Hakim formula. The patients were assigned to four groups according to the quartiles of their PVS measured at each of the three abovementioned timepoints. We examined the association between PVS and all‐cause mortality during the observation period (1134 days). The primary endpoint of this study was all‐cause mortality.
Results
The Kaplan–Meier analysis showed that the high PVS group had a significantly higher mortality rate at admission, transfer to the GW and discharge than the other groups (log‐rank test: P = 0.016, P = 0.005 and P < 0.001, respectively). Univariate Cox regression analysis showed that age, body mass index, history of heart failure, use of beta‐blockers, albumin level, blood urea nitrogen level, N‐terminal pro‐brain natriuretic peptide level and left ventricular ejection fraction were significantly different among the PVS groups and thus were not significant prognostic factors for ADHF. Furthermore, the multivariate analysis revealed that PVS at discharge [hazard ratio (HR) = 1.06 (1.00–1.12), P = 0.048] was an independent poor prognostic factor for ADHF.
Conclusions
This study highlights the effect of PVS measured at different timepoints on the prognoses of ADHF patients. Regular assessment of PVS, particularly at discharge, is crucial for optimising patient management and achieving favourable outcomes in cases of ADHF.
Keywords: acute decompensated heart failure, cardiovascular intensive care unit, long‐term mortality, plasma volume status
Introduction
Plasma volume status (PVS) is a differential marker used for discrepancies between actual and ideal plasma volume (PV). PVS is a prognostic marker for patients with heart failure (HF), 1 , 2 and the validity of actual PV has been confirmed. 3 Actual PV is derived from the haematocrit value and weight of a patient compared with the patient's directly measured PV using the curve fitting technique:
where haematocrit is a fraction, a is 1530 in men and 864 in women and b is 41 in men and 47.9 in women. Using body weight, the ideal PV is calculated using this well‐established formula:
where c is 39 in men and 40 in women.
Relative PVS, an index of the degree of ideal PV deviation, is calculated using the Kaplan–Hakim formula:
Elevated PVS at admission is associated with a worse prognosis in acute decompensated heart failure (ADHF). 4 , 5 , 6 , 7 , 8 However, the effect of PVS measured at different timepoints during hospitalisation on the prognoses of patients with HF, particularly those admitted to cardiovascular intensive care unit (CICU), remains unclear.
Methods
This was a retrospective, observational cohort study of consecutive patients with ADHF admitted to the CICU at Nippon Medical School Hospital between 1 January 2018 and 31 December 2020.
This study was approved by the Institutional Review Board of our institute (Reference No. B‐2021‐433) and conducted according to the revised Declaration of Helsinki. The requirement of written informed consent was waived, as this study is observational. We used an opt‐out method for participant recruitment.
Overall, 363 consecutive patients with ADHF admitted to the hospital CICU were included. Patients who received red blood cell transfusions, underwent haemodialysis, died in the hospital, underwent emergency cardiac surgery, were transferred to another hospital or were discharged directly from the CICU were excluded (Figure 1).
Figure 1.
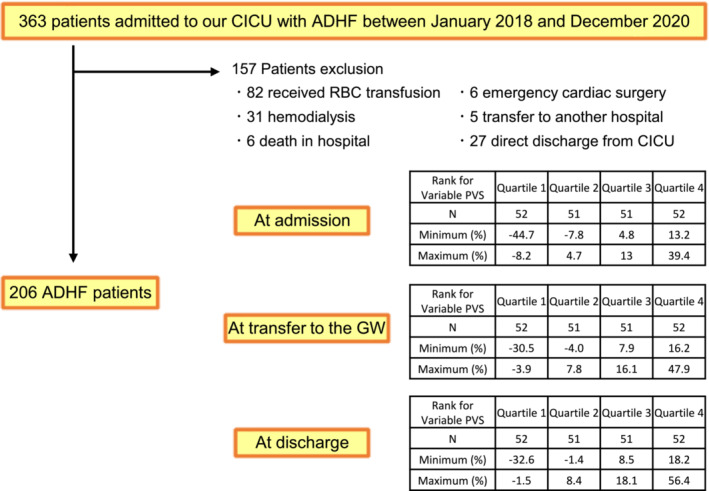
Flow chart of patient inclusion. The patients were divided into quartiles based on their plasma volume statuses (PVSs) at three timepoints: admission, transfer to the general ward (GW) and discharge. They were categorised into Quartiles 1, 2, 3 and 4, with Quartile 1 representing the highest PVS group. ADHF, acute decompensated heart failure; CICU, cardiovascular intensive care unit; RBC, red blood cell.
We measured the patients' PVS at admission, transfer to the general ward (GW) and discharge using the Kaplan–Hakim formula. Patients were categorised into four groups according to the quartiles of their PVS values measured at each timepoint. The association between PVS and all‐cause mortality during the observation period (1134 days) was also examined.
Data on patient demographics, medical history, clinical presentation, including vital signs, blood sampling findings and left ventricular ejection fraction (LVEF), length of hospital stay and clinical outcomes were extracted from patients' medical records. The primary endpoint of this study was all‐cause mortality.
All continuous and categorical variables are expressed as mean ± standard deviation or number and percentage. Student's t‐test and Fisher's exact test were used to analyse continuous and categorical variables, respectively. The four groups classified by quartiles were compared using one‐way analysis of variance. The mortality rate was estimated using the Kaplan–Meier method, and survival estimates were compared using the log‐rank test. A two‐sided P‐value < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS software Version 27 (IBM, Armonk, NY, USA).
Results
In total, 206 patients were analysed. Table 1 shows group clinical characteristics stratified according to discharge PVS quartiles. No significant differences were observed in sex, systolic blood pressure, reduced LVEF (<40%), CICU stay length, hospital stay length, medical history (except HF), oral medical therapy before admission (except beta‐blockers), discharge blood urea nitrogen (BUN) levels or discharge creatinine levels. The high PVS group (Q3 + Q4) was significantly older and had a lower body weight and body mass index (BMI) than the other groups. Regarding blood tests, the high PVS group (Q3 + Q4) exhibited significantly lower haemoglobin, haematocrit and albumin levels at discharge but showed significantly higher N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels at discharge than the other groups. Table S1 shows blood test and weight data at admission and GW transition for each group, according to the quartiles of PVS at discharge. The mean PVS trends at admission, transfer to the GW and discharge were 2.75 ± 13.99%, 6.87 ± 14.66% and 8.26 ± 14.25%, respectively (Figure S1). The number of deaths (survival rates) during the observation period for the four groups divided by PVS at admission was 0 (100%) in Q1, 4 (89.6%) in Q2, 7 (62.4%) in Q3 and 8 (35.1%) in Q4. Similarly, at transfer to the GW, the number of deaths (survival rates) was 0 (100%) in Q1, 4 (89.2%) in Q2, 5 (63.5%) in Q3 and 10 (32.1%) in Q4. At discharge, the number of deaths (survival rates) was 1 (97.8%) in Q1, 1 (97.4%) in Q2, 8 (46.3%) in Q3 and 9 (34.8%) in Q4. Of the 19 deaths, 10 were due to cardiovascular causes, 2 were attributed to infectious diseases and the remaining 7 were due to other causes. Kaplan–Meier analysis showed that the high PVS group (Q3 + Q4) had a significantly higher mortality rate at admission, transfer to the GW and discharge than the other groups (log‐rank test: P = 0.016, P = 0.005 and P < 0.001, respectively; Figure 2A–C). Univariate Cox regression analysis showed that age [hazard ratio (HR) = 1.05 (1.00–1.10), P = 0.040] and PVS at admission [HR = 1.07 (1.03–1.12), P = 0.001], transfer to the GW [HR = 1.07 (1.03–1.12), P < 0.001] and discharge [HR = 1.09 (1.04–1.13), P < 0.001] were significant poor prognostic factors for ADHF (Table 2) and revealed that age, BMI, history of HF, use of beta‐blockers, albumin level, BUN level, NT‐proBNP level and LVEF, which were significantly different among the groups, were not significant prognostic factors for ADHF. Furthermore, multivariate Cox regression analysis indicated that only PVS at discharge was an independent risk factor associated with all‐cause mortality in patients with ADHF after adjusting for age and haemoglobin level at discharge [HR = 1.06 (1.00–1.12), P = 0.048; Table 2]. PVS at admission or transfer to the GW was not an independent risk factor for all‐cause mortality in patients with ADHF after adjusting for age and haemoglobin level at discharge [HR = 1.04, 95% confidence interval (CI) (0.98–1.10), P = 0.193 and HR = 1.04, 95% CI (0.99–1.09), P = 0.111, respectively].
Table 1.
Baseline patient characteristics.
| Variable | All patients (n = 206) | PVS at discharge | P‐value | |||
|---|---|---|---|---|---|---|
| Q1, n = 52 | Q2, n = 51 | Q3, n = 51 | Q4, n = 52 | |||
| PVS < −1.4% | −1.4% ≤ PVS < 8.5% | 8.5% ≤ PVS < 18.2% | 18.2% ≤ PVS | |||
| Age, years | 74.9 ± 12.9 | 66.8 ± 15.0 | 74.9 ± 14.1 | 79.3 ± 8.2 | 74.9 ± 12.9 | <0.001 |
| Male, n (%) | 133 (64.6) | 37 (70.2) | 29 (56.9) | 33 (64.7) | 34 (65.4) | 0.508 |
| Body weight at discharge, kg | 55.8 ± 13.8 | 68.6 ± 13.8 | 55.9 ± 9.5 | 53.7 ± 10.6 | 45.0 ± 9.0 | <0.001 |
| BMI, kg/m2 | 23.9 ± 5.0 | 26.6 ± 5.9 | 24.3 ± 4.9 | 23.2 ± 3.0 | 20.4 ± 2.9 | <0.001 |
| Systolic blood pressure, mmHg | 155.8 ± 39.1 | 164.7 ± 40.5 | 153.8 ± 44.5 | 154.3 ± 35.2 | 150.4 ± 35.6 | 0.284 |
| Reduced LVEF (<40%), n (%) | 127 (61.7) | 37 (71.2) | 31 (60.8) | 31 (60.8) | 28 (53.8) | 0.340 |
| CICU stay length, days | 7.1 ± 5.8 | 6.9 ± 4.9 | 7.7 ± 4.6 | 6.6 ± 5.3 | 7.4 ± 7.9 | 0.692 |
| Hospital stay length, days | 29.9 ± 33.5 | 32.2 ± 53.7 | 26.0 ± 14.8 | 29.1 ± 19.6 | 32.2 ± 32.0 | 0.523 |
| Aetiology of heart failure, n (%) | ||||||
| Ischaemic heart disease | 81 (39.3) | 15 (28.8) | 22 (43.1) | 26 (51.0) | 18 (34.6) | 0.108 |
| Hypertensive heart disease | 40 (19.4) | 16 (30.8) | 3 (5.9) | 9 (17.6) | 12 (23.1) | 0.012 |
| Valvular heart disease | 32 (15.5) | 3 (5.8) | 12 (23.5) | 6 (11.8) | 11 (21.2) | 0.044 |
| TIC | 20 (9.7) | 10 (19.2) | 6 (11.8) | 2 (3.9) | 2 (3.8) | 0.022 |
| Dilated cardiomyopathy | 23 (11.2) | 8 (15.4) | 5 (9.8) | 6 (11.8) | 4 (7.7) | 0.664 |
| Others | 10 (4.9) | 0 (0) | 3 (5.9) | 2 (3.9) | 5 (9.6) | 0.145 |
| Medical history, n (%) | ||||||
| Heart failure | 57 (27.7) | 5 (9.6) | 12 (23.5) | 20 (39.2) | 20 (38.5) | 0.002 |
| Cerebrocardiovascular disease | 76 (36.9) | 2 (3.8) | 7 (13.7) | 6 (11.8) | 8 (15.4) | 0.251 |
| Hypertension | 160 (77.7) | 32 (61.5) | 30 (58.8) | 28 (54.9) | 31 (59.6) | 0.072 |
| Dyslipidaemia | 121 (58.7) | 32 (61.5) | 30 (58.8) | 28 (54.9) | 31 (59.6) | 0.962 |
| Diabetes | 93 (45.1) | 17 (32.7) | 26 (51.0) | 29 (56.9) | 21 (40.4) | 0.051 |
| Medical therapy, n (%) | ||||||
| Beta‐blocker | 80 (38.8) | 12 (23.1) | 21 (41.2) | 26 (51.0) | 21 (40.4) | 0.032 |
| Diuretic | 74 (35.9) | 16 (30.8) | 18 (35.3) | 24 (47.1) | 16 (30.8) | 0.289 |
| ACE‐I/ARB | 84 (40.8) | 17 (32.7) | 21 (41.2) | 27 (52.9) | 19 (36.5) | 0.206 |
| Blood parameters at discharge | ||||||
| Haemoglobin, g/dL | 12.1 ± 2.1 | 14.5 ± 1.5 | 12.4 ± 1.4 | 11.1 ± 1.2 | 10.3 ± 1.1 | <0.001 |
| Haematocrit, % | 36.9 ± 5.8 | 43.6 ± 4.1 | 37.9 ± 3.7 | 34.3 ± 3.5 | 31.5 ± 3.0 | <0.001 |
| Albumin, g/dL | 3.4 ± 0.5 | 3.6 ± 0.4 | 3.5 ± 0.5 | 3.3 ± 0.4 | 3.2 ± 0.5 | <0.001 |
| BUN, mg/dL | 23.5 ± 12.9 | 20.0 ± 9.3 | 24.8 ± 14.9 | 26.6 ± 10.7 | 24.3 ± 14.8 | 0.104 |
| Creatinine, mg/dL | 1.13 ± 0.8 | 1.11 ± 0.6 | 1.11 ± 0.7 | 1.15 ± 0.5 | 1.26 ± 1.1 | 0.382 |
| Log NT‐proBNP, pg/mL | 3.26 ± 0.55 | 2.87 ± 0.56 | 3.33 ± 0.49 | 3.42 ± 0.48 | 3.42 ± 0.50 | <0.001 |
| Echocardiographic findings | ||||||
| LVEF, % | 36.6 ± 16.1 | 31.9 ± 14.4 | 34.2 ± 13.6 | 38.7 ± 15.8 | 41.4 ± 19.9 | 0.012 |
Note: Continuous data are presented as mean ± standard deviation.
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CICU, cardiovascular intensive care unit; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PVS, plasma volume status; Q1, Quartile 1; Q2, Quartile 2; Q3, Quartile 3; Q4, Quartile 4; TIC, tachycardia‐induced cardiomyopathy.
Figure 2.
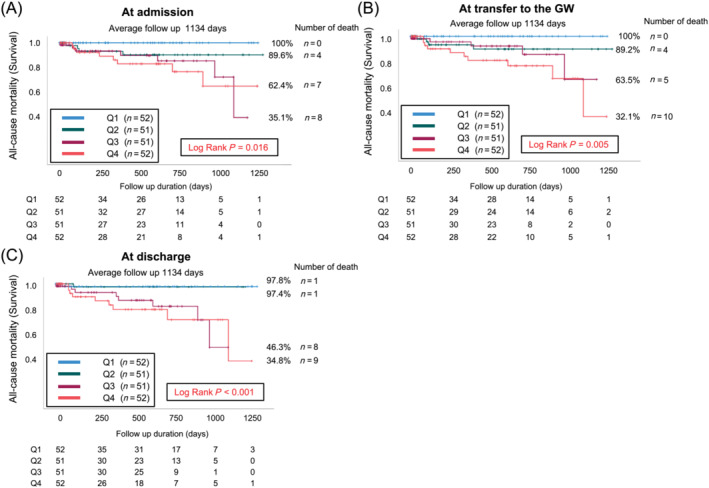
Kaplan–Meier analyses stratified according to the quartiles of the plasma volume statuses of patients with acute decompensated heart failure at (A) admission, (B) transfer to the general ward (GW) and (C) discharge. Q1, Quartile 1; Q2, Quartile 2; Q3, Quartile 3; Q4, Quartile 4.
Table 2.
Cox regression analysis of factors associated with all‐cause mortality in patients with acute decompensated heart failure.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| PVS at admission | 1.07 (1.03–1.12) | 0.001 | ‐ | |
| PVS at transfer to the GW | 1.07 (1.03–1.12) | <0.001 | ‐ | |
| PVS at discharge | 1.09 (1.04–1.13) | <0.001 | 1.06 (1.00–1.12) | 0.048 |
| Age | 1.05 (1.00–1.10) | 0.040 | 1.03 (0.98–1.09) | 0.301 |
| Sex | 0.59 (0.21–1.67) | 0.322 | ‐ | |
| BMI | 0.92 (0.83–1.02) | 0.123 | ‐ | |
| Left ventricular ejection fraction | 1.02 (0.99–1.05) | 0.144 | ‐ | |
| History of heart failure | 2.35 (0.95–5.78) | 0.063 | ‐ | |
| Cerebrocardiovascular disease | 0.04 (0.00–34.74) | 0.355 | ‐ | |
| Use of beta‐blocker | 0.44 (0.17–1.11) | 0.080 | ‐ | |
| Log NT‐proBNP at admission | 2.09 (0.83–5.25) | 0.117 | ‐ | |
| Log NT‐proBNP at discharge | 2.08 (0.87–5.01) | 0.101 | ‐ | |
| Creatinine at discharge | 0.96 (0.55–1.67) | 0.878 | ‐ | |
| Haemoglobin at discharge | 0.60 (0.44–0.82) | 0.001 | 0.79 (0.51–1.23) | 0.299 |
| BUN at discharge | 1.00 (0.97–1.04) | 0.923 | ‐ | |
| Albumin at discharge | 0.46 (0.19–1.11) | 0.085 | ‐ | |
Abbreviations: BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; GW, general ward; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PVS, plasma volume status.
Aims
This study aimed to examine the relationship between PVS measured at various timepoints during hospitalisation and the prognosis of CICU patients with ADHF.
Discussions and conclusions
To our knowledge, this is the first study in which the PVS of patients with ADHF was measured at three specific timepoints (admission, transfer to the GW and discharge) and the correlations between the measured PVS values and patient prognoses were analysed.
The study findings indicate that PVS measured at admission, transfer to the GW and discharge have a significant influence on the prognosis of patients with ADHF. Remarkably, the results highlight that PVS measured at discharge provides the most accurate reflection of prognosis. Consequently, regular assessment of PVS at each of these critical timepoints is clinically important to facilitate optimisation of patient management and successful discharge.
According to the European Society of Cardiology 2021 guidelines, the recommended approach for diagnosing and treating HF with a reduced ejection fraction includes early administration of medications from all four mortality‐lowering drug classes: angiotensin‐converting enzyme inhibitors or angiotensin receptor II blocker‐neprilysin inhibitors, beta‐blockers, mineralocorticoid receptor antagonists and sodium‐glucose‐linked transporter‐2 inhibitors (SGLT2is). 7 Dapagliflozin and empagliflozin reduce the combined risk of worsening HF and cardiovascular death among patients with HF, regardless of their LVEF. 9 , 10 , 11 , 12 We previously reported that empagliflozin effectively reduces body weight and PVS. 12 Lower PVS may improve the prognosis of patients with HF, regardless of their LVEF. However, the extent to which SGLT2is were utilised after discharge was not investigated in the present study. Furthermore, administration of diuretics to individuals with high PVS before discharge from the hospital is not always advisable, as it deteriorates renal function. Regarding clinical implications, the study findings indicate that patients with high PVS at discharge should be closely followed up on an outpatient basis after discharge, and their medications should be more carefully up‐titrated for guideline‐directed medical therapy.
This study has some limitations. First, the overall sample size and number of women in this study were small. Second, outpatient treatment, including comprehensive disease‐modifying pharmacological therapy received after discharge from the hospital, was not considered in the analysis. This limitation restricts our understanding of the long‐term effect of PVS on prognosis. Third, we excluded patients who underwent blood transfusion or haemodialysis. This may have introduced bias and limited the generalisation of the findings to a broader population. Fourth, a previous report has suggested that formula‐based PVS estimation, as used in this study, is not suitable for clinical decision‐making in compensated chronic HF. 13 This study examined PVS up to the time of discharge in hospitalised patients with non‐compensated acute HF and found that the usefulness of PVS as a prognostic factor is limited. Fifth, we focused solely on patients admitted to the CICU and did not consider patients with HF who presented directly to the hospital. Such cases should be included in future studies for a more representative sample.
In conclusion, this study highlights the significant effect of PVS measured at different timepoints on the prognosis of patients with ADHF. Regular assessment of PVS, particularly at discharge, is crucial for optimising patient management and achieving favourable outcomes in ADHF cases.
Funding
None.
Conflict of interest statement
None declared.
Supporting information
Figure S1. Time‐course of change in the plasma volume status from admission to discharge. GW, general ward.
Table S1. Blood parameters and body weight at admission and the time of transfer to the general ward for all patients, stratified according to quartiles of PVS at discharge.
Acknowledgements
The authors extend their sincere thanks to all the people involved in patient care, including emergency staff, technicians, medical engineers, nurses, pharmacists and physicians at Nippon Medical School Hospital.
Hoshika, Y. , Kubota, Y. , Nishino, T. , Shiomura, R. , Shibuya, J. , Nakata, J. , Miyachi, H. , Tara, S. , Iwasaki, Y.‐K. , Yamamoto, T. , and Asai, K. (2024) Prognostic impact of plasma volume status during hospital admission in patients with acute decompensated heart failure. ESC Heart Failure, 11: 1995–2000. 10.1002/ehf2.14874.
References
- 1. Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail 2015;17:35‐43. doi: 10.1002/ejhf.193 [DOI] [PubMed] [Google Scholar]
- 2. Grodin JL, Philips S, Mullens W, Nijst P, Martens P, Fang JC, et al. Prognostic implications of plasma volume status estimates in heart failure with preserved ejection fraction: Insights from TOPCAT. Eur J Heart Fail 2019;21:634‐642. doi: 10.1002/ejhf.1407 [DOI] [PubMed] [Google Scholar]
- 3. Hakim RM. Plasmapheresis. In: Daugirdas JT, Blake PG, Ing TS, eds. Handbook of Dialysis. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2001:236. [Google Scholar]
- 4. Yoshihisa A, Abe S, Sato Y, Watanabe S, Yokokawa T, Miura S, et al. Plasma volume status predicts prognosis in patients with acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care 2018;7:330‐338. doi: 10.1177/2048872617690889 [DOI] [PubMed] [Google Scholar]
- 5. Tamaki S, Yamada T, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, et al. Prognostic value of calculated plasma volume status in patients admitted for acute decompensated heart failure—A prospective comparative study with other indices of plasma volume. Circ Rep 2019;1:361‐371. doi: 10.1253/circrep.CR-19-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shirakabe A, Asai K, Kobayashi N, Okazaki H, Matsushita M, Shibata Y, et al. Prognostic value of both plasma volume status and nutritional status in patients with severely decompensated acute heart failure. CJC Open 2019;1:305‐315. doi: 10.1016/j.cjco.2019.10.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen X, Lin G, Dai C, Xu K. Effect of estimated plasma volume status and left atrial diameter on prognosis of patients with acute heart failure. Front Cardiovasc Med 2023;10:1069864. doi: 10.3389/fcvm.2023.1069864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 9. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020;323:1353‐1368. doi: 10.1001/jama.2020.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413‐1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 11. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451‐1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 12. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089‐1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 13. Hoshika Y, Kubota Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, et al. Effect of empagliflozin versus placebo on plasma volume status in patients with acute myocardial infarction and type 2 diabetes mellitus. Diabetes Ther 2021;12:2241‐2248. doi: 10.1007/s13300-021-01103-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahlgrim C, Birkner P, Seiler F, Grundmann S, Bode C, Pottgiesser T. Estimated plasma volume status is a modest predictor of true plasma volume excess in compensated chronic heart failure patients. Sci Rep 2021;11:24235. doi: 10.1038/s41598-021-03769-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Time‐course of change in the plasma volume status from admission to discharge. GW, general ward.
Table S1. Blood parameters and body weight at admission and the time of transfer to the general ward for all patients, stratified according to quartiles of PVS at discharge.


