Abstract
Aims
Current understanding of the prognosis for patients with chronic kidney disease (CKD) and overlapping cardio‐renal‐metabolic components, specifically heart failure (HF) and diabetes mellitus (DM), remains limited. While previous studies have explored the interactions between CKD, HF, and DM, they have predominantly focused on cohorts of HF or DM patients. This study aims to fill this gap by investigating the long‐term outcomes and treatment patterns in a cohort of CKD patients, particularly those with coexisting HF and DM.
Methods and results
We analysed data from the Swedish national CKD patient cohort, the Swedish Renal Registry, with a follow‐up period extending up to 10 years. The study examined the risks of all‐cause mortality, major adverse cardiovascular events (MACE)—defined as a composite of non‐fatal myocardial infarction, hospitalization for congestive HF, non‐fatal stroke, or cardiovascular death—and the initiation of kidney replacement therapy (KRT). Analyses were conducted using Cox proportional hazards and competing risk models. Among the 27 647 patients, 48% had CKD alone, 12% had CKD with HF, 27% had CKD with DM, and 13% had CKD with both HF and DM. After 5 years, mortality rates were 23% for patients with CKD, 30% for those with CKD/DM, 54% for CKD/HF, and 55% for CKD/HF/DM. The 10 year absolute risk of MACE was 28% for CKD alone, 35% for CKD/DM, 67% for CKD/HF, and 73% for CKD/HF/DM. The adjusted hazard ratio (HR) for mortality was approximately three times higher in patients with any HF combination, with HRs of 2.57 [95% confidence interval (CI) 2.43–2.71] for CKD/HF and 3.22 (95% CI 3.05–3.39) for CKD/HF/DM, compared with CKD alone. The impact of HF on MACE prognosis was even more pronounced, with adjusted sub‐hazard ratios (SHRs) of 3.33 (95% CI 3.14–3.53) for CKD/HF and 4.26 (95% CI 4.04–4.50) for CKD/HF/DM. Additionally, CKD patients diagnosed with HF were less likely to commence KRT, and the risk of death prior to KRT initiation was roughly twice as high for these groups, with SHRs of 2.05 (95% CI 1.93–2.18) for CKD + HF and 2.43 (95% CI 2.29–2.58) for CKD + HF + DM.
Conclusions
In a cohort of CKD patients, having HF contributes substantially to increased mortality and the risk of MACE, and these patients are less likely to start KRT. These findings highlight the urgent need for targeted therapeutic strategies and management plans for CKD patients, particularly those with concurrent HF, to enhance patient prognosis.
Keywords: Chronic kidney disease, Heart failure, Diabetes mellitus, Prognosis, Cardio‐renal‐metabolic, Outcome
Introduction
Chronic kidney disease (CKD) is a global health burden with a prevalence of 11–13% that constitutes a major and independent risk factor for cardiovascular disease and leads to a shorter lifespan. 1 Multimorbidity among patients with CKD is common, as both a cause and consequence of CKD, and may further increase mortality and result in poor quality of life. 2 , 3 Among the most important conditions in patients with CKD are the presence of heart failure (HF) and diabetes mellitus (DM) 4 , 5 ; the complex interactions between the three are referred to as the cardio‐renal‐metabolic syndrome. 6 Despite prior studies having indicated that the coexistence of any of the other conditions worsens the prognosis 7 , 8 , 9 , 10 and the increasing focus on the treatment opportunities in multidisciplinary guidelines, 11 few studies have focused on the prognosis of the overlapping cardio‐renal‐metabolic components in CKD cohorts. While new therapies such as sodium‐glucose co‐transport 2 (SGLT2) inhibitors have been developed that especially benefit patients in this intersection, studies among patients with earlier CKD stages indicate that already recommended HF treatments, such as renin‐angiotensin‐aldosterone system inhibitors (RAASis), are under‐prescribed in this population. 12 Understanding the magnitude of the cardio‐renal‐metabolic impact on the prognosis in CKD populations is essential. The aim of our study was to investigate the long‐term prognosis and treatment patterns in a cohort of CKD patients with overlapping cardio‐renal‐metabolic subgroups in the era before newer therapies were available to patients with kidney dysfunction. For this purpose, we analysed data from a large national cohort of patients with CKD, with follow‐up for almost a decade.
Methods
Study population
The Swedish Renal Registry—Chronic Kidney Disease (SRR‐CKD) is a national Swedish quality control register where patients at Swedish nephrology units with chronic kidney disease non‐dialysis (CKD‐ND) are included. 13 SRR‐CKD has been described in more detail in previous publications 14 ; in short, all referred patients with an incident estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 are required to be included in the register, but nephrology units could also include CKD‐ND patients with a higher eGFR, as long as it is done systematically for all patients. Virtually all (96%) of the nephrology units in Sweden contribute to the register; a recent validation confirmed patient coverage of 95–97%. 15 For this study, we included all patients ≥18 years old with an eGFR < 60 mL/min/1.73 m2 who were included in SRR‐CKD between 1 January 2005 and 1 June 2017. We excluded patients with previous kidney replacement therapy (KRT) before inclusion. At inclusion in the SRR‐CKD, patients were informed and had the right to opt out according to the rules for health care quality registers in Sweden. The study protocol was approved by the ethical review committee in Stockholm, Sweden, and the investigation conforms to the principles outlined in the Declaration of Helsinki.
Covariates
We retrieved data for comorbid conditions, including HF and DM, medication, hospitalization dates, and inpatient and outpatient diagnoses. Each patient's unique social security number was linked to the National Patient Register and the National Prescribed Drug Register 16 at the National Board of Health and Welfare in Sweden. A diagnosis of HF and DM, as well as other comorbid conditions, was defined based on International Classification of Disease 10 (ICD‐10) diagnostic codes prior to inclusion in the SRR‐CKD (Supporting Information, Table S1 ).
From SRR‐CKD, we extracted data regarding age, sex, and type of primary kidney disease. Furthermore, we gathered information regarding clinical characteristics, such as blood pressure, body mass index, and routine laboratory data. Medication was categorized based on the Anatomical Therapeutic Chemical Classification System (ATC) (Supporting Information, Table S2 ) and defined as any dispensed drug from the National Drug Registry within 6 months from baseline or the start of a new follow‐up period during follow‐up (2005–09, 2010–14, and 2015–17). All patients were followed until death or at the end of the observation period.
Outcomes
Data on mortality and cardiovascular outcomes were collected through the linkage with the National Cause of Death Register and the National Patient Register. The primary outcome was all‐cause mortality. Secondary outcomes were major adverse cardiovascular events (MACE) and the start of KRT, defined as the start of dialysis or kidney transplantation. MACE was defined as a composite of a non‐fatal myocardial infarction, hospitalization for congestive HF, non‐fatal stroke, or cardiovascular death. The outcome definitions are listed in Supporting Information, Table S3 .
Statistical analysis
Patient characteristics were presented using means and standard deviations for normally distributed variables and medians and interquartile ranges (IQRs) for non‐normally distributed variables. Categorical variables were presented with proportions. The study population was categorized into four mutually exclusive groups based on the cardio‐renal‐metabolic comorbidities we aimed to study at baseline: CKD without HF and DM (CKD), CKD with HF (CKD + HF), CKD with DM (CKD + DM), and CKD with HF and DM (CKD + HF + DM). The overall survival was graphically described by Kaplan–Meier curves. The incidence rate of both mortality and MACE was computed by dividing the number of events by the person‐time of follow‐up, censoring at the time of kidney transplantation, and non‐cardiovascular death in the follow‐up for MACE. Cumulative incidence plots were produced for the secondary outcomes, MACE and KRT, treating non‐cardiovascular death (MACE) and mortality before KRT as competing risks. The risk for death associated with the different baseline cardio‐renal‐metabolic subgroups was assessed in Cox proportional hazards regression models adjusting for age, sex, and eGFR at baseline. The proportionality assumption was assessed graphically in log–log plots and found to be satisfactory. For the secondary outcomes of MACE and KRT, we performed competing risk regression models (Fine and Gray) 17 using non‐cardiovascular death and mortality before KRT as competing events and adjusting for the same covariates as in the Cox proportional hazards regression models. Missing data (Supporting Information, Table S4 ) for variables included in our adjusted analyses were overall low (<5%), and the analyses were performed on complete cases. Statistical analysis was performed using STATA Version 17 (StataCorp, College Station, TX, USA).
Results
Out of 29 336 patients included in the SRR‐CKD, we excluded n = 27 with an age < 18 years, 1557 with an eGFR > 60 mL/min/1.73 m2 at baseline, and 105 patients with prior KRT or erroneous data input. In our final cohort, there were 27 647 patients, of whom 12 910 (48%) were diagnosed with only CKD at baseline, 3458 (13%) had CKD + HF, 7595 (27%) had CKD + DM, and 3684 (13%) had CKD + HF + DM (Table 1 ). The debut of HF was 2.9 years (IQR 0.8–6.5) prior to inclusion for the CKD + HF group and 3.2 years (IQR 1.0–7.2) for CKD + HF + DM. The debut of DM was 7.2 years (IQR 3.0–11.9) prior to inclusion for the CKD + DM group and 8.1 years (IQR 4.1–11.4) for CKD + HF + DM. Median age was significantly higher for CKD + HF [77 years (IQR 72–84)] and CKD + HF + DM [74 (IQR 68–80)] compared with those with CKD [67 (IQR 59–79)] and CKD + DM [69 (IQR 62–77)].
Table 1.
Overall baseline characteristics in a cohort of patients with chronic kidney disease and cardio‐renal‐metabolic disease
| Number (%), unless indicated | ||||
|---|---|---|---|---|
|
CKD 12 910 (46.7) |
CKD + HF 3458 (12.5) |
CKD + DM 7595 (27.3) |
CKD + HF + DM 3684 (13.3) |
|
| Description [n] | ||||
| Age, median years (IQR) | 67 (59–79) | 77 (72–84) | 69 (62–77) | 74 (68–80) |
| Men | 7993 (61.9) | 2273 (65.7) | 4912 (64.7) | 2430 (66.0) |
| Comorbidity | ||||
| Ischaemic heart disease | 1363 (10.6) | 1432 (41.4) | 1309 (17.2) | 1860 (50.5) [3684] |
| Peripheral arterial disease | 939 (7.3) | 624 (18.1) | 628 (8.3) | 600 (16.3) |
| Cerebrovascular disease | 1761 (13.6) | 830 (24.0) | 1362 (17.9) | 959 (26.0) |
| Atrial fibrillation | 1256 (9.7) | 1677 (48.5) | 850 (11.2) | 1553 (42.2) |
| Hypertension | 11 834 (91.7) | 3404 (98.4) | 7422 (97.7) | 3663 (99.4) |
| Primary kidney disease [n] (%) | ||||
| Polycystic kidney disease | 1000 (7.8) | 78 (2.3) | 99 (1.3) | 14 (0.38) |
| Diabetes nephropathy | n/a | n/a | 3952 (52.1) | 1821 (49.5) |
| Glomerulonephritis | 1869 (14.5) | 175 (5.1) | 323 (4.3) | 63 (1.7) |
| Hypertension | 3384 (26.2) | 1205 (34.9) | 1234 (16.3) | 727 (19.7) |
| Pyelonephritis | 572 (4.4) | 67 (1.9) | 124 (1.6) | 31 (0.84) |
| Renovascular disease | 91 (0.7) | 56 (1.6) | 43 (0.6) | 23 (0.62) |
| CKD, unknown origin | 2619 (20.3) | 876 (25.3) | 977 (12.9) | 525 (14.3) |
| Other specified kidney diseases | 3367 (26.0) | 1000 (28.9) | 840 (11.1) | 478 (13.0) |
| Medication | ||||
| ACE‐Is/ARBs | 7929 (62.9) | 2271 (66.6) | 5888 (78.5) | 2871 (78.7) |
| Beta‐blockers | 6801 (53.9) | 2852 (83.7) | 4942 (65.9) | 3142 (86.1) |
| Calcium channel antagonists | 7239 (57.4) | 1543 (45.3) | 5077 (67.7) | 1937 (53.1) |
| Hypertensive medication, other | 1326 (10.5) | 317 (9.3) | 1130 (15.1) | 511 (14.0) |
| Diuretics | 6692 (53.1) | 3017 (88.5) | 5546 (74.0) | 3426 (93.9) |
| Statins | 5291 (42.0) | 1728 (50.7) | 5323 (71.1) | 2690 (73.7) |
| Mineralocorticoid receptor antagonists | 460 (3.7) | 676 (19.8) | 457 (6.1) | 786 (21.5) |
| Oral antidiabetic medication | 2078 (27.7) | 829 (22.7) | ||
| Insulin | 5045 (67.3) | 2609 (71.5) | ||
| Clinical characteristics | ||||
| Body mass index, median (IQR) | 26.5 (23.2–29.1) | 26.6 (23.4–29.1) | 29.6 (25.4–33.0) | 30.7 (26.4–34.3) |
| Systolic blood pressure, mean (SD) | 140.0 (20.9) | 133.3 (23.4) | 143.4 (22.1) | 136.7 (22.6) |
| Laboratory data | ||||
| eGFR inclusion, mL/min/1.73 m2, mean (SD) | 25.2 (11.1) | 23.5 (9.4) | 24.9 (9.9) | 24.5 (9.4) |
| Albumin/creatinine ratio (mg/g), median (IQR) | 246.9 (47.8–973.5) | 93.8 (24.8–531.0) | 477.9 (78.8–1797.4) | 256.6 (51.3–1396.5) |
| CKD stage at inclusion | ||||
| 3a (eGFR 45–59 mL/min/1.73 m2) | 801 (6.2) | 98 (2.8) | 282 (3.7) | 104 (2.8) |
| 3b (eGFR 30–44 mL/min/1.73 m2) | 2856 (22.1) | 615 (17.8) | 1723 (22.7) | 803 (21.8) |
| 4 (eGFR 15–29 mL/min/1.73 m2) | 6947 (53.8) | 2098 (60.7) | 4385 (57.7) | 2228 (60.5) |
| 5 (eGFR < 15 mL/min/1.73 m2) | 2306 (17.9) | 647 (18.7) | 1205 (15.9) | 549 (17.0) |
ACE‐Is, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HF, heart failure; IQR, interquartile range; SD, standard deviation.
There was a higher prevalence of men compared with women in all four comorbidity groups, with a proportion ranging between 62% and 66%. The median eGFR at inclusion was similar in all groups, ~25 mL/min/1.73 m2. The proportion of patients in different CKD stages was evenly distributed throughout the comorbidity categories, with a majority in CKD stages 4 and 3b, 57% and 22% in all four comorbidity categories (Table 1 ).
Cardiovascular comorbidities were more common in people with HF. Ischaemic heart disease was found in 41% of CKD + HF and 51% of CKD + HF + DM, compared with 11% of CKD and 17% of CKD + DM. Atrial fibrillation was found in 49% of CKD + HF and 42% of CKD + HF + DM, compared with 10% of CKD and 42% of CKD + DM. As expected, diabetes nephropathy was the most common cause of the primary kidney disease in CKD + DM and CKD + HF + DM. About 50% of the patients with DM were judged to have another primary kidney disease by their nephrologist, most commonly hypertension or an unknown kidney disease. Among patients with CKD and CKD + HF, hypertensive kidney disease was most common (Table 1 ). There were no substantial differences with regard to other laboratory measurements between the four comorbidity groups (Supporting Information, Table S5 ).
All‐cause mortality
The overall survival, divided into the subclasses of cardio‐renal‐metabolic syndrome, is presented in Figure 1 . The mortality rate was highest in patients with all three comorbidities, CKD + HF + DM [22.9/100 patient‐years; 95% confidence interval (CI) 21.9–23.8], followed by those with CKD + HF (21.8/100 patient‐years; 95% CI 20.8–22.7). Compared with patients with an HF diagnosis, those with CKD + DM had a more than 50% lower mortality rate (10.1/100 patient‐years; 95% CI 9.8–10.5), while the lowest mortality was among people with only CKD (7.0/100 patient‐years; 95% CI 6.8–7.3) (Table 2 ). The unadjusted risk of death [hazard ratio (HR)] was approximately three times higher in patients with HF: HR 3.23 (95% CI 3.06–3.41) for those with CKD + HF and 3.41 (95% CI 3.23–3.59) for CKD + HF + DM. CKD patients with DM had a 46% higher risk of death compared with CKD patients without DM (HR 1.46; 95% CI 1.39–1.53). After adjustments, the CIs between patients with CKD + HF and CKD + HF + DM widened, but the overall relationship between the different comorbidity categories remained. The absolute risk of death after 3, 5, and 10 years for patients with different cardio‐renal‐metabolic diagnoses showed that the risk was highest for patients belonging to any of the HF groups, with a 3 year mortality risk of 42% and a 5 year mortality of ~55% (Supporting Information, Table S6 and Figure S1 ).
Figure 1.
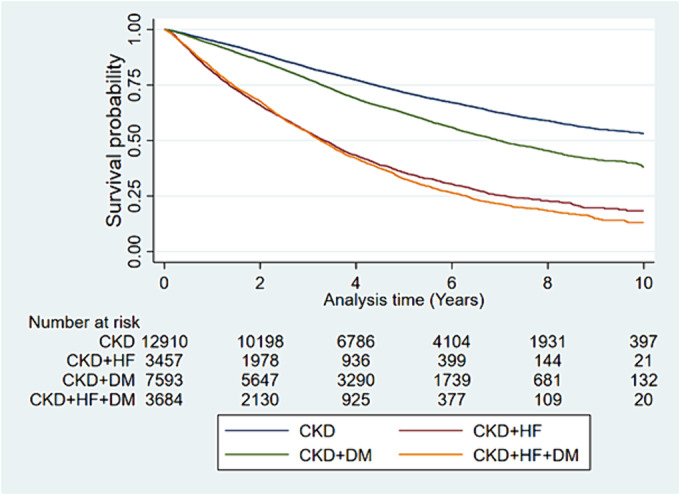
Kaplan–Meier survival plots for all‐cause mortality. CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure.
Table 2.
Survival and major adverse cardiovascular events in people with overlapping cardio‐renal‐metabolic disease
| CKD | CKD + HF | CKD + DM | CKD + HF + DM | |
|---|---|---|---|---|
| All‐cause mortality | ||||
| Incidence rate/100 person‐years (95% CI) | 7.0 (6.8–7.3) | 21.8 (20.8–22.7) | 10.1 (9.8–10.5) | 22.9 (21.9–23.8) |
| Unadjusted hazard ratio (95% CI) | Ref | 3.23 (3.06–3.41) | 1.46 (1.39–1.53) | 3.41 (3.23–3.59) |
| Adjusted hazard ratio (95% CI) a | Ref | 2.57 (2.43–2.71) | 1.55 (1.47–1.62) | 3.27 (3.10–3.45) |
| Major adverse cardiovascular events | ||||
| Incidence rate/100 person‐years | 6.6 (6.4–6.8) | 32.7 (31.4–34.1) | 11.3 (10.9–11.7) | 39.5 (38.0–41.0) |
| Unadjusted sub‐hazard ratio (95% CI) (n = 27 587) | Ref | 4.25 (4.02–4.49) | 1.61 (1.54–1.69) | 4.94 (4.69–5.20) |
| Adjusted sub‐hazard ratio (95% CI) a | Ref | 3.33 (3.14–3.53) | 1.57 (1.50–1.65) | 4.26 (4.04–4.50) |
CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; Ref, reference.
Adjusted for age, gender, and estimated glomerular filtration rate.
Risk of major adverse cardiovascular events and kidney replacement therapy
In line with the overall results for mortality, there was an increased risk for MACE in all cardio‐renal‐metabolic groups, with the highest incidence rate of MACE in the groups with an HF diagnosis (incidence rate 32.7/100 patient‐years; 95% CI 31.4–34.1 for CKD + HF and 39.5/100 patient‐years; 95% CI 38.0–41.0 for CKD + HF + DM) compared with patients with CKD + DM and CKD (11.26/100 patient‐years; 95% CI 10.9–11.7 and 6.58/100 patient‐years; 95% CI 6.4–6.8, respectively) (Table 2 ). These results were also reflected in the cumulative incidence of MACE for all four cardio‐renal‐metabolic groups (Figure 2 ). In the adjusted analyses, the risk of MACE was highest in CKD patients diagnosed with both HF and DM [sub‐hazard ratio (SHR) 4.26; 95% CI 4.04–4.50], followed by CKD patients with HF (HR 3.33; 95% CI 3.14–3.53) and CKD patients with DM (HR 1.57; 95% CI 1.50–1.65) (Table 2 ).
Figure 2.
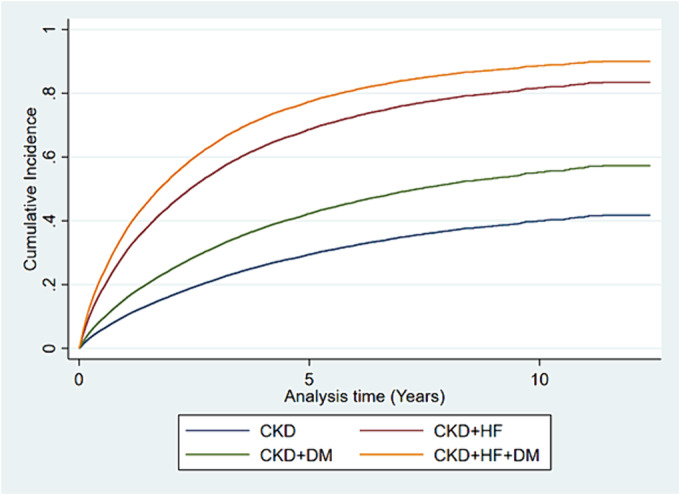
Adjusted cumulative incidence of major adverse cardiovascular events. CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure.
When investigating the interaction of age and sex in the risk for MACE, both interactions were found statistically significant (P‐value < 0.001). There was a higher risk of MACE in patients below 65 years of age for patients with CKD + HF/CKD + HF + DM as compared with older individuals above 65 years of age. Our results also indicated a worse prognosis for women in all cardio‐renal‐metabolic subgroups (Supporting Information, Table S7 ).
Prescribed drugs at inclusion and over follow‐up
RAASis with angiotensin‐converting enzyme inhibitors (ACE‐Is) or angiotensin II receptor blockers (ARBs) were more widely used among patients with diabetes: 79% for both CKD + DM and CKD + HF + DM vs. 63% for CKD and 67% for CKD + HF. The distribution of ACE‐I/ARB use was similar through stages of CKD, apart from CKD stage 5, where the use was slightly lower (Supporting Information, Table S8 ). Beta‐blockers were more common in patients with HF: 84% and 86% for CKD + HF and CKD + HF + DM, respectively, vs. 66% for CKD + DM and 54% for CKD. Overall, mineralocorticoid receptor antagonists (MRAs) were sparsely used, reaching ~20% in patients with HF as compared with <5% in the other groups. Treatment with statins was more common in patients with diabetes. Among diabetic patients, insulin was the dominant form of diabetes treatment. Oral diabetes drugs were used only in 28% (CKD + DM) and 23% (CKD + HF + DM) (Table 1 ).
Over the follow‐up period, the use of the different categories of prescribed drugs was relatively constant in 2005–09, 2010–14, and 2014–17. The use of newer therapies for DM, such as glucagon‐like peptide 1 (GLP1) analogues, was not common; only 2.7% and 3.7% of the patients with CKD + DM and CKD + HF + DM, respectively, were prescribed GLP1 in the most recent cohort (2014–17). The use of SGLT2 inhibitors was almost non‐existent, occurring in only 11 patients in total in 2014–17 (Supporting Information, Table S9 ).
Start of kidney replacement therapy
The risk of starting dialysis or KRT was found to be lower in CKD patients with HF relative to those without HF (Table 3 ). However, in those with DM or DM + HF, the risk for KRT initiation was higher than in patients without any of these comorbidities: SHR 1.37 (95% CI 1.30–1.45) for CKD + DM and SHR 1.18 (95% CI 1.10–1.28) in patients with CKD + HF + DM. The risk of death before the start of KRT was approximately twice as high in people diagnosed with HF: SHR 2.05 (95% CI 1.93–2.18) for CKD + HF and SHR 2.43 (95% CI 2.29–2.58) for CKD + HF + DM (Table 3 ).
Table 3.
Risk of kidney replacement therapy and death before kidney replacement therapy
| CKD |
CKD + HF SHR (95% CI) |
CKD + DM SHR (95% CI) |
CKD + HF + DM SHR (95% CI) |
|
|---|---|---|---|---|
| KRT start | ||||
| Incidence rate/100 person‐years (95% CI) | 8.1 (7.8–8.3) | 5.8 (5.3–6.3) | 10.1 (9.7–10.5) | 9.4 (8.7–10.0) |
| Unadjusted sub‐hazard ratio (95% CI) | Ref | 0.50 (0.45–0.55) | 1.16 (1.10–1.22) | 0.79 (0.73–0.85) |
| Adjusted sub‐hazard ratio (95% CI) a | Ref | 0.73 (0.66–0.81) | 1.37 (1.30–1.45) | 1.18 (1.10–1.28) |
| Death before KRT | ||||
| Death rate/100 person‐years (95% CI) | 6.6 (6.3–6.8) | 20.9 (20.0–22.0) | 8.6 (8.3–9.0) | 21.3 (20.3–22.3) |
| Unadjusted sub‐hazard ratio (95% CI) | Ref | 3.25 (3.06–3.45) | 1.22 (1.15–1.29) | 2.98 (2.80–3.16) |
| Adjusted sub‐hazard ratio (95% CI) a | Ref | 2.05 (1.93–2.18) | 1.28 (1.21–1.35) | 2.43 (2.29–2.58) |
CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; KRT, kidney replacement therapy; Ref, reference; SHR, sub‐hazard ratio.
Adjusted for age, gender, and estimated glomerular filtration rate.
Discussion
In this large, nationwide observational CKD cohort, our analysis unequivocally demonstrates that HF, compared with DM, emerges as a significant determinant of mortality within the CKD population. Patients with CKD and concomitant HF experienced a marked increase in the risk of death, with our findings showing an approximately three‐fold elevation in the 3 year absolute risk of mortality. This underscores the lethal impact of HF on patients already burdened with CKD. Moreover, our study sheds light on the significant risk of MACE among CKD patients with HF, further emphasizing the cardiovascular vulnerability of this population. The high incidence of MACE in patients with both CKD and HF not only corroborates the known cardiovascular implications of CKD but also signals an amplified risk profile brought on by the synergy of CKD with HF. Such findings advocate for a more aggressive and proactive approach to managing cardiovascular risk factors in this cohort. A particularly concerning observation from our study is the increased likelihood of death before the initiation of KRT in patients with CKD and HF. Furthermore, our data indicate a potential undertreatment of HF in the CKD population, as evidenced by the lower prescription rates of RAASis among patients with HF compared with those with DM. This finding points to a missed opportunity in utilizing evidence‐based therapies to mitigate the progression of HF and improve outcomes in CKD patients.
Previous studies of cardio‐renal‐metabolic outcomes
Although advancements in treatment options for patients with cardio‐renal‐metabolic disease exist, few previous studies have analysed their relative prognosis and the overlap between them in a CKD cohort. Despite CKD being a prevalent condition in the general population, existing evidence has also predominantly focused on cohorts with either HF or DM. 21 , 22 , 23 , 24 , 25 A large multinational study with 1.2 million patients with DM type 2 from several clinical cohorts, including primary health care, studied outcomes after the development of cardiovascular and renal diseases. 18 The study concluded that patients diagnosed with CKD and/or HF during the follow‐up had an increased risk of both all‐cause and cardiovascular death. In line with our results, patients with cardiorenal syndrome had three times the higher risk of all‐cause death and almost four times the higher risk of cardiovascular death compared with patients with DM type 2, free of cardiovascular and renal disease. Furthermore, two HF studies based on cohorts from Singapore and the United Kingdom showed increased risk estimates for all‐cause death, cardiovascular death, and hospitalization for cardiovascular disease when adding a CKD and/or DM diagnosis. Similarly to the cohorts with DM, having all three conditions was associated with the worst prognosis. 19 , 20 In another large cohort of HF patients from the Swedish HF registry, the presence of CKD stages 3b–5 was strongly associated with substantially higher mortality. The risk remained after adjusting for DM. 21 More recently, a sub‐analysis of the Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial demonstrated that HF patients with multimorbidity (DM, CKD, or atherosclerotic cardiovascular disease) were at higher risk of all‐cause mortality and cardiovascular death. 22 Interestingly, it was the overlapping conditions with CKD that demonstrated the highest risk of their primary outcome, a composite of cardiovascular death and worsening of HF events. Even though the mean age of DELIVER and our cohort was comparable, the absolute risk of all‐cause death was ~50% lower in DELIVER compared with those with cardio‐renal‐metabolic disease in our cohort, likely reflecting the large impact of reduced kidney function on the overall prognosis.
The interaction of age and sex
In our study, there was a significant effect modification with age and sex. Although younger patients (<65 years) had a lower absolute risk of developing MACE, the relative risk attributed to comorbid conditions, especially HF, was substantially higher. This highlights the severity of developing multimorbidity at a younger age and underlines the importance of early diagnosis and treatment. Adding an HF and/or DM diagnosis was also associated with a strikingly higher risk for women compared with men. Whether the higher risk associations for women are due to lower prescription rates of cardio‐renal‐metabolic treatments or are caused by biological factors remains unknown.
Complexity of the cardio‐renal‐metabolic syndrome
The reason for a worse prognosis in CKD patients with HF is still unclear and likely multifactorial due to the complexity and severity of the cardio‐renal‐metabolic syndrome, which leads to the progression of both HF and CKD. The interaction between CKD, HF, and DM is complex. Some common denominators for the cardio‐renal‐metabolic syndrome include hypertension, neurohormonal and inflammatory responses, and oxidative stress. 23 DM may lead to the progression of HF through microvascular and macrovascular complications and changes in myocardial structure, resulting in diabetic cardiomyopathy. 24 Vascular complications in DM could also affect the kidneys through a combination of pathways, including altered tubulo‐glomerular feedback, tubular hypertrophy, and hypoxia. 25 The CKD and HF interaction involves bidirectional pathways as well as more kidney‐specific factors, such as an increase in serum phosphorus and fibroblast growth factor 23 (FGF‐23) associated with uraemia and cardiovascular disease. 26 Neurohormonal factors result in activation of the RAAS, which leads to sodium and water retention, vasoconstriction, increased blood pressure, and oxidative stress. Inflammation and oxidative stress also play an important role, causing endothelial dysfunction, which impairs vasodilation, increases vascular resistance, and reduces kidney perfusion, leading to decreased cardiac output and impaired kidney function. The above‐described pathophysiological processes may all aggravate each other, leading to worsening of the conditions and increased mortality. 27 , 28
Treatment is a challenge
Treatment of patients with cardio‐renal‐metabolic disease is challenging, especially for those with severe kidney dysfunction. Guidelines stemming from randomized controlled trials are missing because patients with severe CKD are often excluded, 29 and there are limited data to support treatment of HF with RAASis in CKD stages 4–5. 30 Many of the guideline‐recommended drugs, such as ACE‐Is/ARBs or angiotensin receptor‐neprilysin inhibitors (ARNIs), aldosterone antagonists, and metformin, could also have acute effects on kidney function or lead to toxicity and, especially in patients with HF, result in acute kidney injury and/or hyperkalaemia. 31 , 32 This may cause early termination of the drugs, resulting in undertreatment with a negative impact on the long‐term prognosis. 14 The challenge of finding a suitable treatment is also reflected in our results. The mean systolic blood pressure was higher than recommended, 33 especially in patients with only CKD and CKD + HF. Furthermore, given the strong evidence for RAASis, the proportion of patients treated with ACE‐Is/ARBs was quite low across all groups and remained constant over the observation period. The proportion of patients prescribed ACE‐Is/ARBs was highest for the subgroups of patients with DM, indicating that DM or albuminuria was the more common reason for treatment instead of HF. There was also an overall low proportion of patients prescribed statins, especially in patients without DM, despite guidelines recommending treatment with statins for all patients aged >50 years with eGFR < 60 mL/min/1.73 m2. 34
More recently, SGLT2 inhibitors have been shown to effectively lower the risk of cardiovascular events, including hospitalization for HF and kidney failure progression, in patients with severely decreased eGFR and heart failure with preserved ejection fraction (HFpEF). 35 , 36 In DELIVER, patients with increasingly overlapping cardio‐renal‐metabolic conditions were those that benefited the most from being treated with the SGLT2 inhibitor dapagliflozin. 22 Together with other promising therapeutic alternatives for DM type 2 patients with CKD, such as GLP1 agonists and non‐steroidal mineralocorticoid receptor antagonists (nsMRAs), there are now treatments with the potential to substantially improve the prognosis of patients with cardio‐renal‐metabolic disease. 37 , 38 , 39 Given the gap between evidence‐based therapies and the observed prevalence of already available medications in our study, it is still a challenge to implement new knowledge into treatment practices.
Strengths and limitations
Our study has several strengths. While the association between CKD, HF, and DM is recognized, our research provides novel insights into the dynamics and impacts of these comorbidities within a cohort of patients with moderately severe CKD. The study cohort included the majority of all nephrology‐referred CKD patients in a country with complete follow‐up over more than 10 years and without any loss of follow‐up due to the linkage to national health care data sources. Sweden is a country with tax‐funded health care, including generously subsidized drug prescriptions, resulting in low bias from access to health care. One notable strength is also the homogeneity observed among the groups at baseline. All four groups had similar eGFR levels at inclusion. Additionally, the distribution of metabolic and laboratory abnormalities was evenly balanced across the groups. Our study is not without limitations. Although the CKD diagnosis and KRT initiation were validated through the health care quality registry, there is always a risk for some misclassification of the other diagnoses derived from the national health care databases. Furthermore, the use of ICD‐10 codes did not allow us to categorize HF into phenotypes, although HFpEF is usually more commonly found in patients with CKD and is likely part of the pathogenesis. 40
Data on the New York Heart Association (NYHA) functional classification were not available. In our analyses, we chose to describe the characteristics of the cohorts rather than adjusting for covariates such as albuminuria, which we assessed to be the result of the underlying condition(s) and in the causal pathway. We acknowledge that any differences between the groups might have been influenced by their different access to various treatments, as well as mediating factors such as albuminuria or blood pressure. Last, information regarding ethnicity was not available because it is against Swedish law to register; this could limit the generalizability of our findings to other, more diverse populations.
Conclusions
In conclusion, our study clearly shows the distinct impact of HF compared with DM on mortality and MACE risks in CKD populations, with HF emerging as a particularly lethal condition. With the lack of evidence‐based treatment guidelines, there is a risk of possibly undertreating these patients, further complicating their management. Patients with CKD and HF have the worst prognosis but are more often excluded from lifesaving therapies such as KRT. Our findings provide a crucial baseline for future research into novel treatments like SGLT2 inhibitors, which have the potential to significantly improve outcomes, especially for those battling the deadly combination of CKD and HF. This calls for intensified efforts to tackle the challenges of managing CKD with HF, aiming to markedly enhance patient care and survival.
Conflict of interest
J.Ö. has received grants for lectures from AstraZeneca. E.A.‐S. reports no conflict of interest. M.E. reports payment for advisory boards and lectures from Astellas, AstraZeneca, and Vifor Pharma. P.B. has received an honorary lecture from Fresenius 2021 and co‐authored a paper sponsored by Astellas. I.H.L. has received grants for lectures from AstraZeneca, Orion Pharma, and Actelion.
Funding
This work was supported by grants from Stockholm City Council (ALF Medicine; Stockholms Läns Landsting) (grant number FoUI‐974143), the Center for Innovative Medicine (CIMED) (grant number FoUI‐963364), and Njurfonden (The Kidney Fund) Sweden (grant number F2022‐0030).
Supporting information
Table S1. ICD‐codes for comorbidity
Table S2. ATC‐codes for medication
Table S3. Definition of outcomes
Table S4. Amount and frequency of missing variables by variable group
Table S5. Extended clinical characteristics
Table S6. Absolute risk of all cause death and MACE
Table S7. Subgroup analysis, risk of MACE
Table S8. The use of ACE/ARB in the different groups based on CKD‐stage. Numbers in %.
Table S9. Medications used 2009, 2014, 2017
Figure S1. Cumulative incidence curve of absolute risk of death
Österman, J. , Al‐Sodany, E. , Haugen Löfman, I. , Barany, P. , and Evans, M. (2024) Heart failure: the grim reaper of the cardio‐renal‐metabolic triad. ESC Heart Failure, 11: 2334–2343. 10.1002/ehf2.14810.
References
- 1. Collaboration GBDCKD . Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020;395:709‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fraser SDS, Taal MW. Multimorbidity in people with chronic kidney disease: Implications for outcomes and treatment. Curr Opin Nephrol Hypertens 2016;25:465‐472. [DOI] [PubMed] [Google Scholar]
- 3. Sullivan MK, Carrero JJ, Jani BD, Anderson C, McConnachie A, Hanlon P, et al. The presence and impact of multimorbidity clusters on adverse outcomes across the spectrum of kidney function. BMC Med 2022;20:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roger VL. Epidemiology of heart failure: A contemporary perspective. Circ Res 2021;128:1421‐1434. [DOI] [PubMed] [Google Scholar]
- 5. Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat Rev Endocrinol 2016;12:616‐622. [DOI] [PubMed] [Google Scholar]
- 6. Whaley‐Connell A, Sowers JR. Basic science: Pathophysiology: The cardiorenal metabolic syndrome. J Am Soc Hypertens 2014;8:604‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cherney DZI, Repetto E, Wheeler DC, Arnold SV, MacLachlan S, Hunt PR, et al. Impact of cardio‐renal‐metabolic comorbidities on cardiovascular outcomes and mortality in type 2 diabetes mellitus. Am J Nephrol 2020;51:74‐82. [DOI] [PubMed] [Google Scholar]
- 8. Gavina C, Carvalho DS, Dias DM, Bernardo F, Martinho H, Couceiro J, et al. Premature mortality in type 2 diabetes mellitus associated with heart failure and chronic kidney disease: 20 years of real‐world data. J Clin Med 2022;11:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ritchie C, Ekundayo OJ, Muchimba M, Campbell RC, Frank SJ, Liu B, et al. Effects of diabetes mellitus in patients with heart failure and chronic kidney disease: A propensity‐matched study of multimorbidity in chronic heart failure. Int J Cardiol 2009;134:330‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta‐analysis. Eur Heart J 2014;35:455‐469. [DOI] [PubMed] [Google Scholar]
- 11. Handelsman Y, Anderson JE, Bakris GL, Ballantyne CM, Beckman JA, Bhatt DL, et al. DCRM Multispecialty Practice Recommendations for the management of diabetes, cardiorenal, and metabolic diseases. J Diabetes Complications 2022;36:108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swartling O, Yang Y, Clase CM, Fu EL, Hecking M, Hödlmoser S, et al. Sex differences in the recognition, monitoring, and management of CKD in health care: An observational cohort study. J Am Soc Nephrol 2022;33:1903‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Svenskt Njurregister (Swedish Renal Registry) . Årsrapport 2018. www.snronline.se. Accessed 23 November 2022. Region Jönköping, Jönköping, Sweden; 2019.
- 14. Fu EL, Evans M, Clase CM, Tomlinson LA, van Diepen M, Dekker FW, et al. Stopping renin‐angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: A nationwide study. J Am Soc Nephrol 2021;32:424‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Regidor D, McClellan WM, Kewalramani R, Sharma A, Bradbury BD. Changes in erythropoiesis‐stimulating agent (ESA) dosing and haemoglobin levels in US non‐dialysis chronic kidney disease patients between 2005 and 2009. Nephrol Dial Transpl 2011;26:1583‐1591. [DOI] [PubMed] [Google Scholar]
- 16. Wettermark B, Zoëga H, Furu K, Korhonen M, Hallas J, Nørgaard M, et al. The Nordic prescription databases as a resource for pharmacoepidemiological research—A literature review. Pharmacoepidemiol Drug Saf 2013;22:691‐699. [DOI] [PubMed] [Google Scholar]
- 17. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496‐509. [Google Scholar]
- 18. Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GCM, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: A large multinational cohort study. Diabetes Obes Metab 2020;22:1607‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaur P, Saxena N, You AX, Wong RCC, Lim CP, Loh SY, et al. Effect of multimorbidity on survival of patients diagnosed with heart failure: A retrospective cohort study in Singapore. BMJ Open 2018;8:e021291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawson CA, Seidu S, Zaccardi F, McCann G, Kadam UT, Davies MJ, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClinicalMedicine 2021;32:100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Löfman I, Szummer K, Hagerman I, Dahlström U, Lund LH, Jernberg T. Prevalence and prognostic impact of kidney disease on heart failure patients. Open Heart 2016;3:e000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ostrominski JW, Thierer J, Claggett BL, Miao ZM, Desai AS, Jhund PS, et al. Cardio‐renal‐metabolic overlap, outcomes, and dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. J Am Coll Cardiol HF 2023. [DOI] [PubMed] [Google Scholar]
- 23. Valensi P, Prevost G, Pinto S, Halimi JM, Donal E. The impact of diabetes on heart failure development: The cardio‐renal‐metabolic connection. Diabetes Res Clin Pract 2021;175:108831. [DOI] [PubMed] [Google Scholar]
- 24. Braunwald E. Diabetes, heart failure, and renal dysfunction: The vicious circles. Prog Cardiovasc Dis 2019;62:298‐302. [DOI] [PubMed] [Google Scholar]
- 25. DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat Rev Nephrol 2021;17:319‐334. [DOI] [PubMed] [Google Scholar]
- 26. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat Rev Nephrol 2016;12:610‐623. [DOI] [PubMed] [Google Scholar]
- 27. Kumar U, Wettersten N, Garimella PS. Cardiorenal syndrome: Pathophysiology. Cardiol Clin 2019;37:251‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ronco C, Bellasi A, Di Lullo L. Cardiorenal syndrome: An overview. Adv Chronic Kidney Dis 2018;25:382‐390. [DOI] [PubMed] [Google Scholar]
- 29. Decker E, Kendrick J. Research in the CKD clinic: Highs and lows. Adv Chronic Kidney Dis 2014;21:344‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beldhuis IE, Lam CSP, Testani JM, Voors AA, Van Spall HGC, ter Maaten JM, et al. Evidence‐based medical therapy in patients with heart failure with reduced ejection fraction and chronic kidney disease. Circulation (New York, NY) 2022;145:693‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Secora AM, Shin JI, Qiao Y, Alexander GC, Chang AR, Inker LA, et al. Hyperkalemia and acute kidney injury with spironolactone use among patients with heart failure. Mayo Clin Proc 2020;95:2408‐2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whittaker CF, Miklich MA, Patel RS, Fink JC. Medication safety principles and practice in CKD. Clin J Am Soc Nephrol 2018;13:1738‐1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012;2:377‐381. [Google Scholar]
- 34. Wanner C, Tonelli M, the Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group M . KDIGO Clinical Practice Guideline for Lipid Management in CKD: Summary of recommendation statements and clinical approach to the patient. Kidney Int 2014;85:1303‐1309. [DOI] [PubMed] [Google Scholar]
- 35. Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat Med 2021;27:1954‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR‐Preserved trial. Circulation 2021;144:1284‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur Heart J 43:474‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baigent C, Emberson J, Haynes R, Herrington WG, Judge P, Landray MJ, et al. Impact of diabetes on the effects of sodium glucose co‐transporter‐2 inhibitors on kidney outcomes: Collaborative meta‐analysis of large placebo‐controlled trials. Lancet (British edition) 2022;400:1788‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Granata A, Maccarrone R, Anzaldi M, Leonardi G, Pesce F, Amico F, et al. GLP‐1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: State of the art. Clin Kidney J 2022;15:1657‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Löfman I, Szummer K, Evans M, Carrero JJ, Lund LH, Jernberg T. Incidence of, associations with and prognostic impact of worsening renal function in heart failure with different ejection fraction categories. Am J Cardiol 2019;124:1575‐1583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD‐codes for comorbidity
Table S2. ATC‐codes for medication
Table S3. Definition of outcomes
Table S4. Amount and frequency of missing variables by variable group
Table S5. Extended clinical characteristics
Table S6. Absolute risk of all cause death and MACE
Table S7. Subgroup analysis, risk of MACE
Table S8. The use of ACE/ARB in the different groups based on CKD‐stage. Numbers in %.
Table S9. Medications used 2009, 2014, 2017
Figure S1. Cumulative incidence curve of absolute risk of death


