Abstract
Background
Metabolic disorders and inflammatory bowel diseases (IBD) have captured the globe during Westernisation of lifestyle and related dietary habits over the last decades. Both disease entities are characterised by complex and heterogeneous clinical spectra linked to distinct symptoms and organ systems which, on a first glimpse, do not have many commonalities in clinical practice. However, experimental studies indicate a common backbone of inflammatory mechanisms in metabolic diseases and gut inflammation, and emerging clinical evidence suggests an intricate interplay between metabolic disorders and IBD.
Objective
We depict parallels of IBD and metabolic diseases, easily overlooked in clinical routine.
Design
We provide an overview of the recent literature and discuss implications of metabolic morbidity in patients with IBD for researchers, clinicians and healthcare providers.
Conclusion
The Western lifestyle and diet and related gut microbial perturbation serve as a fuel for metabolic inflammation in and beyond the gut. Metabolic disorders and the metabolic syndrome increasingly affect patients with IBD, with an expected negative impact for both disease entities and risk for complications. This concept implies that tackling the obesity pandemic exerts beneficial effects beyond metabolic health.
Keywords: INFLAMMATORY BOWEL DISEASE, NONALCOHOLIC STEATOHEPATITIS, CHRONIC LIVER DISEASE
WHAT IS ALREADY KNOWN ON THIS TOPIC
Epidemiological studies indicate the parallel rise of metabolic disorders and inflammatory bowel disease (IBD).
Experimental studies demonstrated that Westernisation of diet is fuelling metabolic disorders and gut inflammation.
Clinical studies demonstrated a link between metabolic disorders, and foremost obesity, on the risk for developing IBD and a poor disease course.
WHAT THIS STUDY ADDS
We review and discuss recent clinical and experimental research with a focus on clinical implications.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We provide an overview of current knowledge which may help researchers, clinicians and policymakers to guide decision making.
Introduction
The incidence and prevalence of metabolic disorders such as obesity, the metabolic syndrome (MS) and inflammatory bowel diseases (IBD) increased during the past decades. In 1997, the WHO has called out an obesity epidemic, and about a decade later researchers introduced the MS pandemic.1 Today, food safety and the control of the obesity epidemic still rank highly on the agenda of the WHO, because the situation deteriorated during the last 20 years. The World Obesity Atlas 2023 reported that 38% of the world population are affected by overweight or obesity, and the Diabetes Atlas reported in 2021 that 537 million people are living with diabetes.2 3 IBD is expected to affect 1 in 100 individuals in certain populations in the next years, especially in Western countries, and newly industrialised countries show similar developments.4 5 As such, the rise of metabolic conditions and IBD occurred during Westernisation of lifestyle and particularly the diet,4 6 and an overlap of metabolic diseases and IBD is expected to be observed more frequently in daily practice tomorrow. Recent developments also convey a substantial socioeconomic burden for healthcare providers in the future and impose clinical implications for patients that should be considered by their doctors.
Clinical background
The MS is defined by the International Diabetes Federation as central obesity (measured by waist circumference considering ethnic differences) plus any two of the following: raised blood pressure (≥130 mm Hg systolic or ≥85 mm Hg diastolic or treatment), raised triglycerides (≥150 mg/dL or treatment), raised fasting plasma glucose (≥100 mg/dL or diagnosed type 2 diabetes (T2D)) or reduced high-density lipoprotein cholesterol (<40 mg/dL in males and <50 mg/dL in females) (box 1).2 Many definitions have appeared in the past decades from various international organisations and, despite minor differences, it became clear that the MS and associated morbidities, such as cardiovascular risk, reflect one of the most common disease entities conveying substantial morbidity.7 Although the current definition of the MS does not consider metabolic dysfunction-associated steatotic liver disease (MASLD), it is evident that metabolic liver disease goes hand in hand with the MS and is involved in some aspects, for example, systemic low-grade inflammation.8 9
Box 1. The metabolic syndrome.
The development of the metabolic syndrome (MS) involves genetic risk, Western behavioural and dietary patterns and gut microbial perturbation, all known to impinge on a key element: central obesity. Central obesity brings along insulin resistance and systemic low-grade inflammation, which contribute to the complex pathophysiology of the MS.170 171 For example, overnutrition instigates the expansion of adipocytes, causing local hypoxia172 and the infiltration of adipose tissue by immune cells. This leads to the secretion of proinflammatory cytokines (eg, tumour necrosis factor (TNF), interleukin 6 (IL-6)) and suppression of anti-inflammatory adiponectin production, culminating in metabolic dysfunction and insulin resistance.173–175 The dysfunctional adipose tissue also increases circulating free fatty acids (FFA), driving lipotoxicity in liver and muscle tissue176 which further promotes insulin resistance and steatotic liver disease (metabolic dysfunction-associated steatotic liver disease, MASLD). Perturbation of the intestinal microbiota may lead to a disruption of the intestinal barrier, causing the systemic translocation of bacterial metabolites fuelling low-grade inflammation and metabolic diseases.75 According to the International Diabetes Federation, the MS is defined as having central obesity (measured by waist circumference considering ethnic differences) plus any two of the following: raised blood pressure (≥130 mm Hg systolic or ≥85 mm Hg diastolic or treatment), raised triglycerides (≥150 mg/dL or treatment), raised fasting plasma glucose (≥100 mg/dL or diagnosed type 2 diabetes) or reduced high-density lipoprotein (HDL) cholesterol (<40 mg/dL in males and <50 mg/dL in females). Notably, insulin resistance and MASLD are no feature of this definition, although these phenotypes come along frequently with the MS and contribute to metabolic disease.
IBD reflects a complex and heterogeneous chronic immune-mediated condition that typically (but not exclusively) affects the gut. Inflammatory gastrointestinal symptoms usually involve diarrhoea, pain and bloody stools, and inflammatory lesions may affect the entire gastrointestinal tract from the oral cavity to the anus (in Crohn’s disease (CD)), conveying a heterogenous clinical picture. Moreover, extraintestinal disease manifestations in active or quiescent IBD most commonly affect the skin and joints, but may virtually be observed in any organ system (box 2).10 11
Box 2. Inflammatory bowel diseases.
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), describes a condition of chronic remittent episodes of inflammation in and beyond the gastrointestinal tract. The complex nature of immune dysregulation virtually affects every immune cell compartment in IBD while only a minority of cases can be explained by genetics.177–179 Recent evidence indicates that Westernisation of life significantly contributes to the development and rising incidence of IBD across the globe. Experimental studies implicate that particular dietary constituents and excess of macronutrients, such as sugar, fat and food additives, trigger or worsen experimental gut inflammation in mice, as further laid out in figure 2. These food constituents either directly affect mucosal immunity or indirectly dysregulate immune responses by affecting (metabolic) functions of the gut microbiota. Collectively, a current concept of IBD pathophysiology depicts a scenario in which specific nutrients in a Western diet trigger or worsen gut inflammation in IBD, while few nutritional intervention trials indicate a direct role for the diet on the disease course.
Epidemiology
A systematic analysis for the Global Burden of Disease Study 2017 reported that the prevalence of IBD increased from 1990 to 2017 across the globe, with highest numbers observed in high-income countries such as North America.12 Hospitalisation rates for IBD are still increasing in Asia, Eastern Europe and Latin America, whereas they seem to stabilise in North America, Western Europe and Oceania.13 Notably, the number of children and adolescents with IBD is continuously rising, as, for example, monitored in Canada,14 and a clear trend in rising incidence and prevalence has been documented across the globe.15 A similar development has been documented for metabolic diseases in children and adolescents, for example, 3% of children and 5% of adolescents worldwide exhibited the MS in 2020.16 Likewise, the prevalence of the MS in adults has reached rates up to 12–31%, which largely depends on the disease definition and the study population.17 Similarly, the prevalence of T2D, a consequence of insulin resistance in the MS, dramatically increased in many parts of the world, for example, Australia, during the last two decades,18 and today 1 in 10 individuals are living with diabetes.2
The parallel rise of IBD and metabolic disorders (with the MS being the most pronounced phenotype) raises the possibility that these disorders originate from similar culprits in Westernised societies, such as a Western lifestyle with related dietary habits and microbial rarefication.19 Moreover, the high prevalence of metabolic disease entities raises the probability of concomitant MS (or other metabolic disorders) in patients with IBD, warranting studies that define clinical consequences.
Common backbone
Some evidence points towards a mutual relationship of IBD with metabolic diseases as summarised in figure 1. Although little clinical overlap between metabolic diseases and IBD exists in terms of symptoms, both are frequently characterised by non-communicable tissue inflammation, for example, in adipose tissue (in obesity) or gut tissue (in IBD). Moreover, both diseases are frequently characterised by systemic low-grade inflammation (picked up by clinical routine testing of high-sensitive C reactive protein and blood count) and they share a phenotype that is not picked up by clinical routine measurements, that is, perturbation of the gut microbial ecosystem (which promotes susceptibility to develop disease in model systems).20 As such, metabolic disorders such as obesity, T2D and MASLD show considerable similarities with IBD pathophysiology, which could possibly be explained by environmental factors that contribute to the development of metabolic disorders and IBD alike.21 22 More specifically, Westernisation of lifestyle involves sedentary habits, accumulation of risk factors (eg, smoking, antibiotic use and hygiene measures) and Westernisation of diet.23 The Western diet is characterised by increased intake of calories derived from sugar (eg, simple carbohydrates) and fat (eg, long-chain saturated or ω-6 polyunsaturated fatty acids), and typically involves intake of processed foods with or without food additives (eg, emulsifiers, food colourants), while dietary fibre intake is reduced.6 Indeed, populations across the world have experienced the emergence of obesity, the MS and IBD when adopting this lifestyle.24 However, not only the phenotypic parallels and epidemiological studies support the notion of a similar culprit that lies within Westernisation of life, which would explain the parallel rise of IBD and metabolic disorders. Also, experimental evidence in model systems indicated shared mechanisms of inflammation, partly involving gut microbial perturbation, as summarised in figure 2. Collectively, phenotypic, epidemiological and experimental evidence indicates that a common backbone of IBD and metabolic disorders is formed by Westernisation of life and particularly the diet. Here, we review the clinical link between IBD and metabolic disorders and discuss clinical implications.
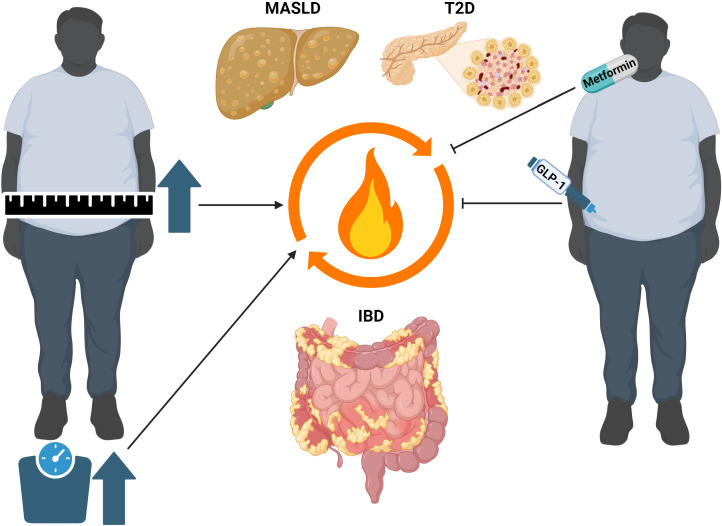
Figure 1.
Clinical link between obesity and metabolic disorders with inflammatory bowel disease (IBD). Obesity and associated metabolic disorders such as metabolic dysfunction-associated steatotic liver disease (MASLD) and type 2 diabetes (T2D) seem to negatively influence the course of IBD. For example, visceral adiposity is associated with risk for flares in ulcerative colitis (UC) and Crohn’s disease (CD),38 and steatotic liver disease serves as an independent risk factor for the development of CD.51 In turn, the diagnosis of IBD increases the risk of developing MASLD and liver fibrosis when compared with the general population.48 Moreover, the treatment with antidiabetic agents with proven effects in metabolic diseases seems to reduce the risk for developing IBD64 and disease burden in patients with IBD.65
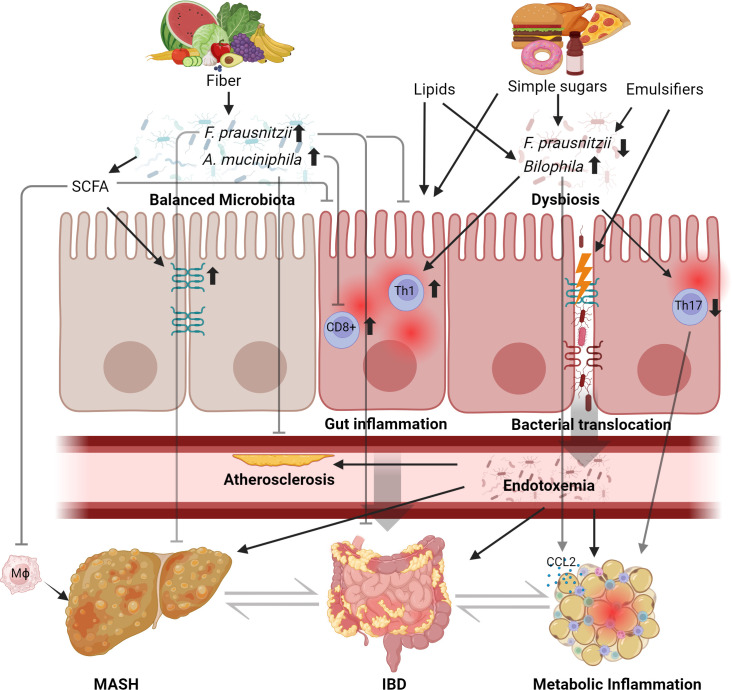
Figure 2.
Mechanisms of diet-induced metabolic inflammation in mice. Studies in rodents demonstrated that dietary patterns and perturbation of the commensal microbiota affect susceptibility to gut inflammation and metabolic diseases, in humans exemplified by inflammatory bowel disease (IBD) and metabolic dsyfunction-associated steatohepatitis (MASH). A healthy diet, for example, a plant-based diet rich in fibres, supports the growth of arguably beneficial commensals such as Faecalibacterium prausnitzii and other commensals that produce short-chain fatty acids (SCFA).180 The diet and microbial commensalism may promote health or disease by modulation of the immune system. For example, liver injury and insulin resistance decreased in high-fat diet-fed mice supplemented with F. prausnitzii 181 and F. prausnitzii attenuated experimental colitis.182 Microbial SCFA protects against experimental liver disease and macrophage infiltration,183 increases gut barrier function184 and ameliorates experimental gut inflammation .185 Similarly, supplementation of Akkermansia muciniphila (which vanishes in Western microbiomes) attenuates endotoxaemia-induced metabolic inflammation and atherosclerosis186 and ameliorates colitis severity by preventing the infiltration of CD8+ T cells.187 By contrast, a Western diet containing, for example, dietary emulsifiers, simple carbohydrates (sugar) and long-chain fatty acids and cholesterol (lipids) may worsen intestinal inflammation and promote metabolic disorders,76 in part by alteration and function of the commensal gut microbiota.71 188 189 For example, a Western diet perturbs gut barrier dysfunction and evokes endotoxaemia fuelling metabolic inflammation in and beyond the gut of mice.186 190 IBD, inflammatory bowel disease.
Clinical link between metabolic disorders and IBD
Obesity and IBD
The prevalence of obesity and IBD has risen globally over the last decades. Overweight was defined by the WHO as a body mass index (BMI) of >25 kg/m2 while obesity is defined by a BMI of >30 kg/m2, while cut-offs vary by ethnicity.25 It was recognised that obesity is associated with more severe IBD, for example, complicated perianal CD,26 27 and a meta-analysis of clinical trials in IBD performed between 1991 and 2008 demonstrated a gradual increase in average weight along with disease activity over time.28 A similar rise of obesity rates was observed in paediatric IBD populations, with ~20% of patients with CD and ~30% of patients with ulcerative colitis (UC) being obese in the USA.29 Studies from the past years have investigated whether obesity differentially affects CD compared with UC. For example, a dose–response meta-analysis in adults demonstrated a strong association of BMI with the risk for developing CD, while no association was found for UC.30 Other studies have also reported that obesity preferentially predisposes to CD, but not to UC, when assessing five prospective cohorts with 601 009 participants and 563 incident cases of CD and 1047 cases of UC.31 A large cohort study from Korea investigated a potential relationship between waist-hip circumference and risk for IBD in >10 million participants from the 2009 national health screening programme and 9.3 years of follow-up. Incidence rates were 2.11 for CD and 8.4 for UC per 100 000 person-years and CD was again associated with degree of abdominal obesity.32 Notably, obesity rates in IBD seem to increase over the last years, when assessing almost 40 000 patients with IBD from a commercial database in the USA. Obesity was reported in 37.3% of patients with IBD, with high rates of metabolic comorbidities.33 Likewise, a recent study from France reported rates of overweight and obesity in 24.1% and 12.2% of patients with IBD.34 In turn, according to an analysis with a large healthcare database from the USA, bariatric surgery or weight loss medications lowered the risk for developing IBD35; however, a similar study demonstrated the opposite effect.36 Collectively, these studies indicate that obesity and related comorbidities are on the rise in patients with IBD (as similarly observed in the general population), and that obesity is a risk factor for the development of CD and complications (such as perianal disease). Two major aspects remain unresolved. First, it is suspected, but not proven, that the parallel rise of both conditions occurred due to a common culprit (and not due to their prevalent nature). Second, despite recent alarming trends, it remains understudied whether increasing rates of obesity impact patient management, treatment responses and surgery rates in patients with IBD.37 For example, obesity might have a direct impact on disease severity in IBD, as exemplified by a recent study suggesting that visceral obesity shortened the interval between IBD flares.38
MASLD interactions with IBD
MASLD, previously termed non-alcoholic fatty liver disease, has emerged globally and reflects a systemic disorder (beyond simple liver steatosis) with numerous metabolic perturbations.39 40 The new nomenclature has been introduced, as it better reflects the metabolic nature as a complex syndrome.41 Notably, the studies discussed below have been performed with variable definitions; however, MASLD and other definitions have yielded highly similar patient phenotypes and outcomes.42 43 It may not be surprising that important interactions between IBD and MASLD are increasingly recognised.44–46 For example, lean patients with IBD exhibited higher MASLD rates when compared with lean non-IBD patients.47 A key study demonstrated that 42% of patients with IBD exhibited MASLD with advanced liver fibrosis in 9.5%,48 and a similar trend in another study.49 Importantly, BMI and T2D prevalence were significantly lower in patients with IBD-MASLD compared with matched non-IBD patients, but IBD appeared as independent risk factor for liver fibrosis, suggesting that chronic gut inflammation contributes to liver pathology.48 This is also in accordance with a report showing that CD poses a higher MASLD risk compared with UC which might be explained by the higher burden of systemic inflammation.50 The intriguing and potentially important clinical aspect of risk for liver fibrosis in IBD needs to be confirmed in further studies. As observed for obesity, MASLD seems to be associated with an increased risk for developing CD, but not UC,51 and presence of MASLD might even affect cardiovascular risk in IBD.52 Moreover, the presence of MASLD (steatosis, but not fibrosis) might increase the risk of an IBD flare.53 Despite these recent clinical observations, the origin of such observations remains unclear, but could involve gut microbial aspects along the gut-liver axis as reported for other liver diseases.54 As for obesity, it remains understudied if MASLD and IBD affect the natural course of both diseases and related complications. For example, the presence of MASLD increased the risk for IBD-related readmission to hospital,55 and in the USA, hospitalised patients with MASLD and IBD exhibited greater morbidity and mortality when compared with patients with IBD (without MASLD).56 Collectively, these studies indicated that patients with IBD are at risk for developing MASLD, which poses a risk for a poor patient outcome.
T2D and IBD
T2D reflects a globally emerging disorder (affecting up to 10% of the population), which conveys features of chronic inflammatory disorders.57 However, the clinical relationship between T2D and IBD is poorly defined. In a nationwide population-based study from Denmark authors observed an increased risk of T2D in patients with CD and UC (when compared with the expected incidence), which increased over the last decades,58 and which appears specific to Europeans.59 Also, data from the UK Biobank support a risk association of CD with T2D,60 but whether the presence of T2D provides a risk for IBD is currently unknown. A preclinical study in obese mice showed that glucose dysregulation worsened degree of experimental colitis and that treatment of hyperglycaemia reversed experimental colitis severity.61 Patients with IBD with concomitant T2D did not show an increased need for biological therapy (when compared with patients with IBD without T2D), but patients with T2D with IBD exhibited systemic inflammation and reported lower quality of life.62 In line, the MS was associated with an inflammatory disease behaviour in CD, which was noted in a cohort comprising 688 patients with IBD from the Prevención con dieta mediterránea (PREDIMED) trial.63 In turn, a recent cohort study from Taiwan suggested metformin, first-line therapy for T2D, reduced the risk for developing IBD.64 A cohort study from Denmark identified an association of GLP-1-based therapies with an improved IBD course (when compared with other antidiabetics), as indicated by a composite score comprising the need for oral corticosteroids, tumour necrosis factor (TNF) inhibitors, IBD-related hospitalisation or IBD-related surgery.65 66 Collectively, the bidirectional interaction between IBD and metabolic diseases warrants further investigation.
Hallmarks of IBD and metabolic disorders beyond clinical praxis
A possible link between IBD and metabolic disorders has already been researched a decade ago.67 Based on experimental evidence, we are increasingly appreciating the metabolic nature of gut inflammation.6 Moreover, metabolomic translational studies in tissue and urine from patients with IBD indeed suggest complex perturbation of organismal metabolism.6 68 In turn, the concept of systemic low-grade inflammation in metabolic diseases and the MS has been established nearly two decades ago, which is considered a key driver of insulin resistance.69 IBDs and metabolic disorders comprise a spectrum of disease manifestations affecting the gut and metabolically active organ systems (boxes 1 and 2) and they conceptually exhibit similar pathophysiological mechanisms that were explored in experimental models. Westernisation of dietary cues instigates or worsens experimental gut inflammation in mice6 and at the same time triggers liver steatosis, metabolic dysregulation and obesity.70 For example, excessive intake of sucrose worsens colitis in genetically susceptible mice,71 similar to excessive intake of fructose, which induces experimental liver steatosis and metabolic dysregulation.72 Moreover, disease models of gut inflammation and obesity show evidence for gut microbial alterations (either evoked by diet or tissue inflammation) which serves as a driving force of inflammatory or metabolic phenotypes,73–77 also in the context of sugar exposure.6 78 Beyond similarities in dietary cues and a dysbiotic microbiota, experimental studies also indicated perturbation of the same cellular mechanisms underlying experimental gut inflammation and metabolic diseases such as obesity, as recently reviewed.6 Moreover, metabolic control partly occurs in the gut, for example, deletion of gut epithelial toll-like receptor 5 induces the MS in mice,79 gut bile acids improve fibre-controlled glucose metabolism80 and gut hormones control organismal metabolism through specialised epithelial actions.81 Collectively, these experimental studies suggest that Westernisation of diet could explain aspects of IBD and metabolic diseases by a mutual pathophysiological backbone that spans across organ systems.
Hallmarks of these experimental findings have been translated to human IBD and metabolic disorders alike. For example, specific constituents in a Western diet have been linked to dysbiosis,82–86 and gut microbial dysbiosis has emerged as a driver of IBD and metabolic disorders such as obesity (figure 2).74 75 87–89 Gut microbial perturbation may exert many functional consequences. For example, bacterial metabolism of tryptophan in the gut is altered in IBD and the MS and has been shown to control experimental gut inflammation and energy metabolism,90 and likewise, tryptophan and related metabolites are implicated in the control of metabolic dysregulation.91 92 Moreover, microbial encroachment in the gut mucosa correlates with metabolic disease aspects, especially in dysglycaemic humans.93
Another clinical parallel between metabolic disorders and IBD in humans is systemic low-grade inflammation, which predicts poor outcome for patients with the MS and IBD alike.94–98 In line with these observations, patients with IBD increasingly develop obesity and metabolic disorders (as reviewed above and exemplified in a medical registry analysed between 2010 and 2019).33 Finally, IBD and metabolic diseases (most notably the MS) share a common denominator impacting human health: cardiovascular complications. Conceptually, systemic inflammation in IBD and the MS contributes to atherosclerotic cardiovascular disease, such as myocardial infarction and stroke.99 100 Collectively, experimental, epidemiological and clinical studies depict intricate parallels that are easily overlooked in clinical routine, paving the way for a concept in which IBD and metabolic diseases share phenotypic disease aspects: Westernised dietary cues, gut microbial perturbation, systemic low-grade inflammation with similar mechanisms and cardiovascular risk (figure 3).
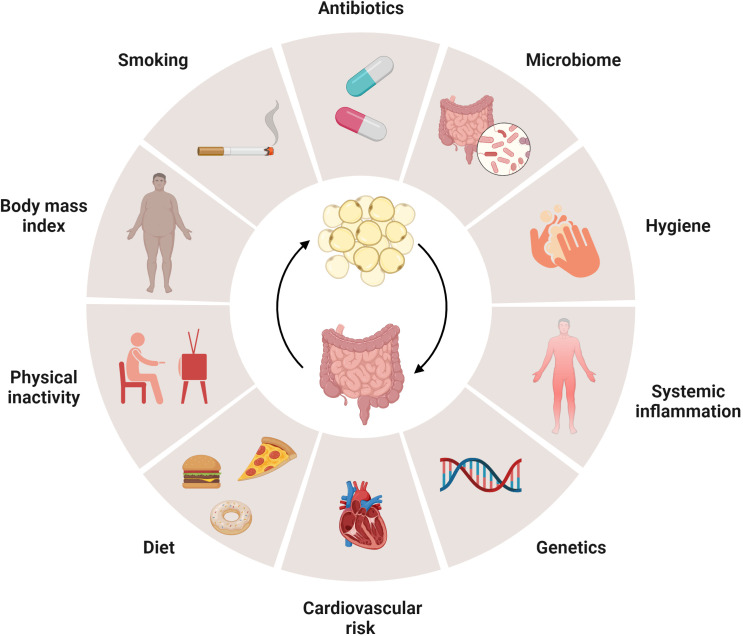
Figure 3.
Metabolic inflammation in obesity and related disorders and in inflammatory bowel disease (IBD). The pathophysiology of metabolic disorders including metabolic syndrome (MS), type 2 diabetes (T2D) or metabolic dysfunction-associated steatotic liver disease (MASLD) shares considerable similarities with IBD, which is supported by experimental studies summarised in figure 2. Westernisation of lifestyle is considered to promote the development of these disease entities as incidence and prevalence are rising worldwide,21 22 with several risk factors that are shared by metabolic diseases and IBD. Experimental models indicate that similar mechanisms, such as gut microbial dysbiosis and nutrient excess, promote metabolic inflammation in obesity and related disorders and IBD alike.6
Clinical implications
As many robust clinical observations demonstrating the interaction between IBD and metabolic disorders only emerged over the last years, clinical trials that directly target the potential relevance of metabolic disorders in IBD (or vice versa) are scarce. However, manifold implications may be inferred by knowledge about disease-relevant aspects and patient management that stem from studies in patients with either condition, which are relevant for gastroenterologists caring for patients with IBD and metabolic disorders.
Morbidity
First, metabolic diseases, and foremost the MS, inflict substantial morbidity to patients with or without IBD, with consequences for individuals and healthcare systems in the next decade. For example, the MS conveys substantial risk for cardiovascular complications, such as stroke or myocardial infarction, and comes along with mortality.101 102 This appears notable because patients with IBD are already at risk for cardiovascular complications,103–105 with an HR for cardiovascular disease of 1.71 in patients with IBD.106 It is also expected that morbidity and mortality will be derived from complications of chronic liver disease in the future, as up to 50% of patients with IBD exhibit MASLD, frequently culminating in cirrhosis and portal hypertension with or without hepatocellular carcinoma.107 Moreover, obese patients with IBD exhibit increased risk for surgical complications, such as infection, conversion to laparotomy and visceral injury.108 Thus, the long-term cardiovascular, hepatic and gastrointestinal consequences of metabolic diseases and their impact on surgical complications should be explained to patients with IBD.
Sarcopenic obesity
Muscle wasting or reduced muscle mass (myopenia) and impaired muscle function in obese patients, referred to as sarcopenic obesity, can be easily overlooked in clinical praxis, although myopenia comes along with morbidity and mortality.109 For example, a retrospective study with 106 patients with CD from the UK demonstrated that myopenia (here, reduced muscle mass assessed by CT) was associated with primary non-response to anti-TNF therapy.110 Moreover, sarcopenia in obesity (reported for ~40% of obese patients with IBD111) may predict the need for IBD-related surgery in a retrospective study with 90 patients from the USA.112 The European Society for Clinical Nutrition and Metabolism (ESPEN) guideline on clinical nutrition in patients with IBD summarises that patients are at risk for muscle wasting (associated with restrictive eating behaviours), which is why low-calorie diets for obese patients with IBD should be used with caution.113 The practical guideline on obesity care in patients with gut and liver diseases recommends screening for sarcopenic obesity with functional and imaging tests, and nutritional status, and assessment in patients with IBD and obesity is recommended before intestinal surgery.114
Cancer
Due to the rising incidence and prevalence of IBD across the globe12 and due to obesity as a risk factor for solid malignancies,31 115 it is expected that the incidence of malignant gastrointestinal complications such as small intestinal116 117 and large intestinal cancers118 will continuously rise during the next decade. A direct link between gut inflammation, obesity and cancer risk has been corroborated by experimental studies (eg, ref 119), and creeping fat in CD contributes to the inflammatory tone.120 Indeed, systemic low-grade inflammation emerges as a pathomechanism in obesity-related malignancies,121 and histological inflammatory activity in IBD associated with risk for colorectal neoplasia (OR: 2.5).122 These findings collectively underline the importance of treating obesity and IBD, because patients are typically young and thus may experience malignant complications.123 International guidelines thus recommend screening for colorectal neoplasia in patients with IBD 6–8 years after symptom onset and further surveillance dependent on the extent of inflammation, family history and comorbidities (eg, primary sclerosing cholangitis (PSC)), while metabolic disorders are not yet considered.
Malnutrition
Today, overnutrition (and its sequelae with obesity) is a common phenomenon and requires treatment as discussed below. The ESPEN guideline recommends screening of nutritional status at IBD diagnosis and during follow-up, especially in hospitalised patients, and screening for the MS in patients with obesity.114 Besides overnutrition, undernutrition and nutrient deficiencies commonly occur in patients with IBD,111 124 which conveys risk of death125 and requires identification, monitoring and treatment.126 For example, micronutrient deficiency (of, for example, folic acid, iron or vitamin B12) should be identified and supplemented in daily praxis to avoid malabsorptive complications and possibly hospitalisation and worsening of disease.124 Updated ESPEN guidelines on clinical nutrition in IBD summarise that a BMI<18.5 kg/m2 is a risk factor for IBD-related complications (eg, fistula or surgical complications) similar to obesity (which provides risk for a disease flare).127 Malnutrition should therefore be assessed regularly and treated, although prospective controlled clinical trials that would demonstrate improved patient outcome are lacking for patients with IBD.126 127
Quality of life
Many studies indicate that quality of life is gradually impaired in patients with increasing obesity and related metabolic disorders such as the MS,128–130 which is similarly true for active IBD.131 132 In patients with IBD and obesity, the quality of life may be even worse and reflects a significant issue, although the study quality appears limited.133 In turn, undernutrition exerts strong effects on the quality of life, with best evidence for the elderly patient (without IBD), which may be improved by correction of malnutrition.134 Therefore, we suggest encouraging discussion about quality of life with the patient to improve compliance and provide motivation to therapy, as medical therapies (similar to surgery) for IBD have been demonstrated to improve the long-term quality of life.135
Worsening of disease
Although prospective clinical trials that would demonstrate a benefit of treating metabolic diseases in IBD are lacking, recent studies indicate that metabolic disorders, such as, for example, obesity, provide risk for developing CD and convey a poor course of established IBD.133 Likewise, it appears that obese patients with IBD show impaired treatment response to biologics, which is understudied, but which could be related to dosing or biological effects of obesity on the inflammatory tone of an organism.136 A recent study shed light on the potential relevance and related mechanisms. In a prospective observational cohort study with 141 adult patients with CD and UC and moderate to severe disease with the need for biological therapy in the USA (CONSTELLATION), Yarur et al report that intra-abdominal visceral fat is associated with poor response to biological therapy (infliximab, vedolizumab or ustekinumab).137 More specifically, patients in higher visceral adipose tissue quartiles (determined by dual-energy X-ray absorptiometry) exhibited poor clinical and endoscopic response to biologics after 30 weeks when compared with patients in the lower quartile, which could not be explained by the drug trough levels. As such, patients with visceral obesity are less likely to achieve remission by biological therapy.
Moreover, treatment of patients with IBD and a GLP-1 agonist or DDP4 inhibitor (for T2D) exhibited reduction in adverse clinical events (defined by a composite of the need for oral corticosteroid treatment, need for TNF inhibitor treatment, IBD-related hospitalisation or IBD-related major surgery), as compared with patients treated with other antidiabetic drugs in a Danish registry.65 However, controlled clinical trials are lacking and GLP-1 agonists display severe gastrointestinal side effects (eg, pancreatitis, bowel obstruction), reflecting uncertainty for patients with IBD.138 Therefore, there are several IBD-related aspects linked to metabolic disorders conveying a poor disease course, which should be explained to the patient. Whether drugs that treat metabolic diseases affect risk for developing IBD or disease outcome warrants controlled clinical trials.66
Patient management
The negative impact of metabolic conditions in IBD on morbidity, malnutrition, quality of life and the disease course (with complications) are arguments for screening and treatment of obesity and related disorders in such patients. This is supported by some evidence suggesting that treatment of metabolic disorders improves patient outcomes (in terms of complications in patients without IBD).65 139–141 We suggest screening regularly for aspects of the MS in obese patients with IBD and considering sarcopenia that can be masked by obesity. Screening of the MS should involve evaluation of BMI, blood glucose, HbA1c, hypertension and dyslipidaemia. Moreover, obese patients with IBD should be evaluated for metabolic liver disease by sonography and elastography and laboratory testing on a reasonable regular basis, while evidence for this approach stems from epidemiological (risk) studies and hepatology guidelines (when liver fibrosis or cirrhosis is present). Moreover, obesity and related disorders provide risk for developing kidney disease and heart failure (particularly with preserved ejection fraction), which should be considered if clinical suspicion is high (eg, hypertension, with pulmonary or peripheral oedema or anaemia).142 143
The European guideline for the management of obesity recommends reducing weight in patients with obesity (5–10% of initial weight), while treatment goals go beyond reduction of body fat.139 143 This is achieved by reduced intake of calories (500-1000 kcal/day; 15–30% of daily calorie intake) by dietary counselling with a nutritionist, physical activity (>150 min/week moderate aerobic exercise), cognitive–behavioural therapy and pharmacotherapy (eg, GLP-1 receptor agonists). Bariatric surgery should be considered in patients with a BMI≥40.0 kg/m2 or with BMI between 35.0 and 39.9 kg/m2 and comorbidities if conventional therapy is ineffective after 6 months.143 For patients with IBD and obesity, it appears plausible that similar recommendations should apply.114 However, this may be challenging for some patients, either because (chronically) active IBD impairs the ability to adopt a calorie-restricted diet (which may worsen sarcopenic obesity) or to adopt a physically active lifestyle. Likewise, it is unclear whether medical therapy for obesity affects gut inflammation (except for orlistat which is not recommended for patients with IBD) in patients with IBD, while the risk of morbidity after bariatric surgery appears slightly increased in patients with IBD.144–147 These aspects led to the formulation of guidelines for obesity in chronic gut and liver diseases, advocating that malnutrition is determined by Global Leadership Initiative on Malnutrition criteria, while medical and surgical therapy is similar when compared with patients without IBD.114
Future aspects
Experimental evidence indicated similar inflammatory mechanisms of metabolic disorders and gut inflammation, forming a basis for the concept of metabolic gut inflammation in IBD.6 In line with this concept, more and more translational and clinical evidence is emerging that the diet and related gut microbial cues contribute to gut inflammation in IBD. Therefore, it may not be surprising that therapeutic concepts including faecal microbiota transplantation or microbe-based therapies are currently being studied to treat IBD148 149 and the MS alike.150 This approach appears warranted because patients with the MS exhibit a dysbiotic gut microbiota that controls insulin resistance.73 151 152 Likewise, the human gut microbiota from patients with IBD deteriorates experimental inflammation in mice153 and faecal microbiota transplantation induces remission in patients with active UC in 26% of patients (when compared with 15% in the control groups), as indicated by a meta-analysis comprising 13 randomised controlled studies and 580 patients analysed.154 Yet, gut-directed microbial therapies are not established in clinical praxis, neither to treat metabolic disorders nor to induce remission in patients with IBD.
Insights into the genetic overlap of genetic risk between metabolic diseases and immune-mediated conditions such as IBD may help to better understand their common backbone. For example, host genetics were linked to the abundance of specific metabolites in the serum of patients with IBD, which may point towards novel metabolic disease mechanisms.155 156 In line, perturbation of cholesterol metabolism has been reported in a small cohort of patients with IBD, and a genetic risk locus for obesity (FTO variant, rs9939609) also provided risk for developing CD.157 In turn, genetic risk (indicated by a score) was associated with reduced weight loss in patients with obesity completing a meal replacement programme.158 How genetic risk stratification can be clinically exploited to manage IBD and metabolic diseases is currently not established in clinical praxis.
Another interesting aspect that emerges from recent advances of our biological understanding of IBD and metabolic diseases is covered by interleukin 23 (IL-23). Anti-IL-23 monoclonal antibodies (eg, risankizumab, mirikizumab) have been recently approved to treat CD and UC, respectively.159 160 Notably, IL-23 ameliorated experimental MS in mice,161 possibly suggesting that selective blockade of this cytokine promotes metabolic disorders in susceptible individuals, a signal which has not yet been reported. Similarly, proinflammatory cytokines such as IL-1 or TNF have long been implicated in the pathogenesis of metabolic disorders such as MASLD or T2D.162–164 Indeed, some smaller studies have demonstrated that targeting IL-1 improves glycaemia in T2D,165 and targeting TNF increased insulin sensitivity in patients with ankylosing spondylitis and psoriasis.166 167 TNF has emerged as a metabolic messenger, such that the role of anti-TNF therapies and also other anti-inflammatory therapies on obesity and related metabolic disorders should be evaluated for patients with IBD in the future.168
Collectively, targeted therapies (probably based on individual genetic risk) and modulation of the gut microbiota reflect valuable approaches to treat metabolic diseases and IBD alike, and the relevance of anti-inflammatory therapies in patients with obesity and IBD deserves to be better explored.
Conclusion
The parallels and intricate link between metabolic disorders and IBD have emerged over the last decade by experimental and clinical studies alike. Current guidelines recommend treatment of obesity and metabolic disorders in patients with IBD as reported for patients without IBD. Many studies raise important questions for the future (box 3). Foremost, many observations support the idea that the parallel rise of IBD and metabolic disorders may be explained by a common culprit, or pattern, that can be found in Westernisation of life and likely Western dietary habits. Dietary guidelines in Europe and the USA clearly acknowledge the effect of a healthy diet and lifestyle. However, authorities also realise that only 50–60% of the population in the USA adhere to those guidelines (which has not changed over the last decade).169 This indicates the growing burden of diet-related diseases in the near future and the need for education. Moreover, we must consider today how to feed the world population by sustainable means, acknowledging the risk of Westernisation. ‘Prevention is better than cure’, thus a change in food policy and preventive measures by governmental programmes are increasingly required to effectively tackle the metabolic pandemic.
Box 3. Outstanding questions.
How does perturbation of metabolism in metabolic disorders promote gut inflammation in inflammatory bowel disease (IBD)?
Does identification and treatment of metabolic disorders and sarcopenic obesity impact the course of IBD?
Can we apply targeted therapy for both metabolic disorders and IBD?
Does inflammatory disease control of IBD reduce the risk for developing metabolic disorders or the disease course?
How can we effectively tackle the rise of metabolic disorders and IBD?
Does prevention of obesity reduce the risk for solid malignancies in IBD?
Do patients with IBD with the metabolic syndrome benefit from tightened cancer surveillance?
Acknowledgments
Figures were created with BioRender.com.
Footnotes
Contributors: TEA, MM, AJ and HT prepared the manuscript and figures. TEA submitted the manuscript.
Funding: This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement n° 101039320) and this research was funded in part by the Austrian Science Fund (FWF) grant DOI: 10.55776 and FG15 (to T.E.A.).
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 2008;28:629–36. 10.1161/ATVBAHA.107.151092 [DOI] [PubMed] [Google Scholar]
- 2. International Diabetes Federation . IDF Diabetes Atlas. 10th edn. Brussels, Belgium, 2021. Available: https://www.diabetesatlas.org [Google Scholar]
- 3. Koliaki C, Dalamaga M, Liatis S. Update on the obesity epidemic: after the sudden rise, is the upward trajectory beginning to flatten Curr Obes Rep 2023;12:514–27. 10.1007/s13679-023-00527-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 5. Kaplan GG, Windsor JW. The four Epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2021;18:56–66. 10.1038/s41575-020-00360-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adolph TE, Meyer M, Schwärzler J, et al. The metabolic nature of inflammatory bowel diseases. Nat Rev Gastroenterol Hepatol 2022;19:753–67. 10.1038/s41575-022-00658-y [DOI] [PubMed] [Google Scholar]
- 7. Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999-2006. Diabetes Care 2011;34:216–9. 10.2337/dc10-0879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamirani YS, Katz R, Nasir K, et al. Association between inflammatory markers and liver fat: the multi-ethnic study of atherosclerosis. J Clin Exp Cardiolog 2014;5:1000344. 10.4172/2155-9880.1000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fu CE, Yong JN, Ng CH, et al. The prognostic value of including non-alcoholic fatty liver disease in the definition of metabolic syndrome. Aliment Pharmacol Ther 2023;57:979–87. 10.1111/apt.17397 [DOI] [PubMed] [Google Scholar]
- 10. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010;28:573–621. 10.1146/annurev-immunol-030409-101225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med 2020;383:2652–64. 10.1056/NEJMra2002697 [DOI] [PubMed] [Google Scholar]
- 12. Alatab S, Sepanlou SG, Ikuta K. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol 2020;5:17–30. 10.1016/S2468-1253(19)30333-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buie MJ, Quan J, Windsor JW, et al. Global hospitalization trends for Crohn’s disease and ulcerative colitis in the 21st century: a systematic review with temporal analyses. Clin Gastroenterol Hepatol 2023;21:2211–21. 10.1016/j.cgh.2022.06.030 [DOI] [PubMed] [Google Scholar]
- 14. El-Matary W, Carroll MW, Deslandres C, et al. The 2023 impact of inflammatory bowel disease in Canada: special populations-children and adolescents with IBD. J Can Assoc Gastroenterol 2023;6:S35–44. 10.1093/jcag/gwad016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuenzig ME, Fung SG, Marderfeld L, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology 2022;162:1147–59. 10.1053/j.gastro.2021.12.282 [DOI] [PubMed] [Google Scholar]
- 16. Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: a systematic review and modelling analysis. The Lancet Child & Adolescent Health 2022;6:158–70. 10.1016/S2352-4642(21)00374-6 [DOI] [PubMed] [Google Scholar]
- 17. Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: a meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract 2022;188:109924. 10.1016/j.diabres.2022.109924 [DOI] [PubMed] [Google Scholar]
- 18. Islam SMS, Siopis G, Sood S, et al. The burden of type 2 diabetes in Australia during the period 1990-2019: findings from the global burden of disease study. Diabetes Res Clin Pract 2023;199:110631. 10.1016/j.diabres.2023.110631 [DOI] [PubMed] [Google Scholar]
- 19. Sonnenburg JL, Sonnenburg ED. Vulnerability of the industrialized Microbiota. Science 2019;366. 10.1126/science.aaw9255 [DOI] [PubMed] [Google Scholar]
- 20. Metwaly A, Reitmeier S, Haller D. Microbiome risk profiles as biomarkers for inflammatory and metabolic disorders. Nat Rev Gastroenterol Hepatol 2022;19:383–97. 10.1038/s41575-022-00581-2 [DOI] [PubMed] [Google Scholar]
- 21. Clemente-Suárez VJ, Beltrán-Velasco AI, Redondo-Flórez L, et al. Global impacts of Western diet and its effects on metabolism and health: a narrative review. Nutrients 2023;15:2749. 10.3390/nu15122749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rizzello F, Spisni E, Giovanardi E, et al. Implications of the westernized diet in the onset and progression of IBD. Nutrients 2019;11:1033. 10.3390/nu11051033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Christ A, Latz E. The Western lifestyle has lasting effects on metaflammation. Nat Rev Immunol 2019;19:267–8. 10.1038/s41577-019-0156-1 [DOI] [PubMed] [Google Scholar]
- 24. Schlesinger S, Neuenschwander M, Schwedhelm C, et al. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr 2019;10:205–18. 10.1093/advances/nmy092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caleyachetty R, Barber TM, Mohammed NI, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol 2021;9:419–26. 10.1016/S2213-8587(21)00088-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blain A, Cattan S, Beaugerie L, et al. Crohn’s disease clinical course and severity in obese patients. Clin Nutr 2002;21:51–7. 10.1054/clnu.2001.0503 [DOI] [PubMed] [Google Scholar]
- 27. Singh S, Dulai PS, Zarrinpar A, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol 2017;14:110–21. 10.1038/nrgastro.2016.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moran GW, Dubeau M-F, Kaplan GG, et al. The increasing weight of Crohn’s disease subjects in clinical trials: a hypothesis-generatings time-trend analysis. Inflamm Bowel Dis 2013;19:2949–56. 10.1097/MIB.0b013e31829936a4 [DOI] [PubMed] [Google Scholar]
- 29. Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:2162–8. 10.1002/ibd.21585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rahmani J, Kord-Varkaneh H, Hekmatdoost A, et al. Body mass index and risk of inflammatory bowel disease: a systematic review and dose-response meta-analysis of cohort studies of over a million participants. Obes Rev 2019;20:1312–20. 10.1111/obr.12875 [DOI] [PubMed] [Google Scholar]
- 31. Chan SSM, Chen Y, Casey K, et al. Obesity is associated with increased risk of Crohn’s disease, but not ulcerative colitis: a pooled analysis of five prospective cohort studies. Clin Gastroenterol Hepatol 2022;20:1048–58. 10.1016/j.cgh.2021.06.049 [DOI] [PubMed] [Google Scholar]
- 32. Je Y, Han K, Chun J, et al. Association of waist circumference with the risk of inflammatory bowel disease: a nationwide cohort study of 10 million individuals in Korea. J Crohns Colitis 2023;17:681–92. 10.1093/ecco-jcc/jjac193 [DOI] [PubMed] [Google Scholar]
- 33. Elangovan A, Shah R, Ali SMJ, et al. High burden of obesity and low rates of weight loss pharmacotherapy in inflammatory bowel disease: 10-year trend. Crohn’s & Colitis 360 2023;5. 10.1093/crocol/otad007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bacha RA, Bouhnik Y, Serrero M, et al. Obesity in adult patients with inflammatory bowel disease: clinical features and impact on disability. A cross-sectional survey from the GETAID. Dig Liver Dis 2023;55:1632–9. 10.1016/j.dld.2023.05.008 [DOI] [PubMed] [Google Scholar]
- 35. Kochhar GS, Desai A, Syed A, et al. Risk of de-Novo inflammatory bowel disease among obese patients treated with bariatric surgery or weight loss medications. Aliment Pharmacol Ther 2020;51:1067–75. 10.1111/apt.15721 [DOI] [PubMed] [Google Scholar]
- 36. Allin KH, Jacobsen RK, Ungaro RC, et al. Bariatric surgery and risk of new-onset inflammatory bowel disease: a nationwide cohort study. J Crohns Colitis 2021;15:1474–80. 10.1093/ecco-jcc/jjab037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gu P, Luo J, Kim J, et al. Effect of obesity on risk of hospitalization, surgery, and serious infection in biologic-treated patients with inflammatory bowel diseases: A CA-IBD cohort study. Am J Gastroenterol 2022;117:1639–47. 10.14309/ajg.0000000000001855 [DOI] [PubMed] [Google Scholar]
- 38. Sehgal P, Su S, Zech J, et al. Visceral adiposity independently predicts time to flare in inflammatory bowel disease but body mass index does not. Inflamm Bowel Dis 2024;30:594–601. 10.1093/ibd/izad111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tilg H, Adolph TE, Dudek M, et al. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab 2021;3:1596–607. 10.1038/s42255-021-00501-9 [DOI] [PubMed] [Google Scholar]
- 40. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015;62:S47–64. 10.1016/j.jhep.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 41. Rinella ME, Lazarus JV, Ratziu V, et al. A Multisociety delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023;79:1542–56. 10.1016/j.jhep.2023.06.003 [DOI] [PubMed] [Google Scholar]
- 42. Song R, Li Z, Zhang Y, et al. Comparison of NAFLD, MAFLD and MASLD characteristics and mortality outcomes in United States adults. Liver Int 2024;44:1051–60. 10.1111/liv.15856 [DOI] [PubMed] [Google Scholar]
- 43. Younossi ZM, Paik JM, Stepanova M, et al. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J Hepatol 2024. 10.1016/j.jhep.2024.01.014 [DOI] [PubMed] [Google Scholar]
- 44. Zou ZY, Shen B, Fan JG. Systematic review with meta-analysis: epidemiology of nonalcoholic fatty liver disease in patients with inflammatory bowel disease. Inflamm Bowel Dis 2019;25:1764–72. 10.1093/ibd/izz043 [DOI] [PubMed] [Google Scholar]
- 45. Principi M, Iannone A, Losurdo G, et al. Nonalcoholic fatty liver disease in inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis 2018;24:1589–96. 10.1093/ibd/izy051 [DOI] [PubMed] [Google Scholar]
- 46. Sourianarayanane A, Garg G, Smith TH, et al. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis 2013;7:e279–85. 10.1016/j.crohns.2012.10.015 [DOI] [PubMed] [Google Scholar]
- 47. Martínez-Domínguez SJ, García-Mateo S, Gargallo-Puyuelo CJ, et al. Inflammatory bowel disease is an independent risk factor for metabolic dysfunction-associated steatotic liver disease in lean individuals. Inflamm Bowel Dis 2023. 10.1093/ibd/izad175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rodriguez-Duque JC, Calleja JL, Iruzubieta P, et al. Increased risk of MAFLD and liver fibrosis in inflammatory bowel disease independent of classic metabolic risk factors. Clin Gastroenterol Hepatol 2023;21:406–14. 10.1016/j.cgh.2022.01.039 [DOI] [PubMed] [Google Scholar]
- 49. Martínez-Domínguez SJ, García-Mateo S, Gargallo-Puyuelo CJ, et al. Crohn's disease is an independent risk factor for liver fibrosis in patients with inflammatory bowel disease and non-alcoholic fatty liver disease. Eur J Intern Med 2024;120:99–106. 10.1016/j.ejim.2023.10.019 [DOI] [PubMed] [Google Scholar]
- 50. Kodali A, Okoye C, Klein D, et al. Crohn’s disease is a greater risk factor for nonalcoholic fatty liver disease compared to ulcerative colitis: a systematic review. Cureus 2023;15:e42995. 10.7759/cureus.42995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen J, Dan L, Tu X, et al. Metabolic dysfunction-associated fatty liver disease and liver function markers are associated with Crohn’s disease but not ulcerative colitis: a prospective cohort study. Hepatol Int 2023;17:202–14. 10.1007/s12072-022-10424-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kablawi D, Aljohani F, Palumbo CS, et al. Nonalcoholic fatty liver disease increases cardiovascular risk in inflammatory bowel diseases. Crohn’s & Colitis 360 2023;5. 10.1093/crocol/otad004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hyun HK, Lee HW, Park J, et al. Hepatic steatosis but not fibrosis is independently associated with poor outcomes in patients with inflammatory bowel disease. Gut Liver 2024;18:294–304. 10.5009/gnl220409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tilg H, Adolph TE, Trauner M. Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab 2022;34:1700–18. 10.1016/j.cmet.2022.09.017 [DOI] [PubMed] [Google Scholar]
- 55. Noorian S, Jeon Y, Nguyen MT, et al. The impact of NAFLD on hospitalization outcomes in patients with inflammatory bowel diseases: nationwide analysis. Inflamm Bowel Dis 2022;28:878–87. 10.1093/ibd/izab199 [DOI] [PubMed] [Google Scholar]
- 56. Soni A, Yekula A, Dahiya DS, et al. Influence of Nonalcoholic fatty liver disease on inflammatory bowel disease hospitalizations in the United States. Ann Gastroenterol 2023;36:646–53. 10.20524/aog.2023.0839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature 2019;576:51–60. 10.1038/s41586-019-1797-8 [DOI] [PubMed] [Google Scholar]
- 58. Jess T, Jensen BW, Andersson M, et al. Inflammatory bowel diseases increase risk of type 2 diabetes in a nationwide cohort study. Clin Gastroenterol Hepatol 2020;18:881–8. 10.1016/j.cgh.2019.07.052 [DOI] [PubMed] [Google Scholar]
- 59. Niu Y, Zhang Q, Wei Y. Causal effects of inflammatory bowel disease on risk of type 2 diabetes: a two-sample multivariable Mendelian randomization study. Acta Diabetol 2024. 10.1007/s00592-024-02254-9 [DOI] [PubMed] [Google Scholar]
- 60. Zhu Z, Jia Y, Li F-R, et al. Inflammatory bowel disease and risk of global cardiovascular diseases and type 2 diabetes. Inflamm Bowel Dis 2023. 10.1093/ibd/izad163 [DOI] [PubMed] [Google Scholar]
- 61. Francis KL, Alonge KM, Pacheco MC, et al. Diabetes exacerbates inflammatory bowel disease in mice with diet-induced obesity. World J Gastroenterol 2023;29:4991–5004. 10.3748/wjg.v29.i33.4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Din H, Anderson AJ, Ramos Rivers C, et al. Disease characteristics and severity in patients with inflammatory bowel disease with coexistent diabetes mellitus. Inflamm Bowel Dis 2020;26:1436–42. 10.1093/ibd/izz305 [DOI] [PubMed] [Google Scholar]
- 63. García-Mateo S, Martínez-Domínguez SJ, Gargallo-Puyuelo CJ, et al. Lifestyle can exert a significant impact on the development of metabolic complications and quality life in patients with inflammatory bowel disease. Nutrients 2023;15:3983. 10.3390/nu15183983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tseng CH. Metformin use is associated with a lower risk of inflammatory bowel disease in patients with type 2 diabetes mellitus. J Crohns Colitis 2021;15:64–73. 10.1093/ecco-jcc/jjaa136 [DOI] [PubMed] [Google Scholar]
- 65. Villumsen M, Schelde AB, Jimenez-Solem E, et al. GLP-1 based therapies and disease course of inflammatory bowel disease. EClinicalMedicine 2021;37:100979. 10.1016/j.eclinm.2021.100979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Arvanitakis K, Koufakis T, Popovic D, et al. GLP-1 receptor agonists in obese patients with inflammatory bowel disease: from molecular mechanisms to clinical considerations and practical recommendations for safe and effective use. Curr Obes Rep 2023;12:61–74. 10.1007/s13679-023-00506-3 [DOI] [PubMed] [Google Scholar]
- 67. Gonçalves P, Magro F, Martel F. Metabolic inflammation in inflammatory bowel disease: crosstalk between adipose tissue and bowel. Inflamm Bowel Dis 2015;21:453–67. 10.1097/MIB.0000000000000209 [DOI] [PubMed] [Google Scholar]
- 68. Aldars-García L, Gil-Redondo R, Embade N, et al. Serum and urine metabolomic profiling of newly diagnosed treatment-naïve inflammatory bowel disease patients. Inflamm Bowel Dis 2024;30:167–82. 10.1093/ibd/izad154 [DOI] [PubMed] [Google Scholar]
- 69. Rohm TV, Meier DT, Olefsky JM, et al. Inflammation in obesity, diabetes, and related disorders. Immunity 2022;55:31–55. 10.1016/j.immuni.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee H, Lee IS, Choue R. Obesity, inflammation and diet. Pediatr Gastroenterol Hepatol Nutr 2013;16:143–52. 10.5223/pghn.2013.16.3.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Khan S, Waliullah S, Godfrey V, et al. Dietary simple sugars alter microbial Ecology in the gut and promote colitis in mice. Sci Transl Med 2020;12. 10.1126/scitranslmed.aay6218 [DOI] [PubMed] [Google Scholar]
- 72. Hannou SA, Haslam DE, McKeown NM, et al. Fructose metabolism and metabolic disease. J Clin Invest 2018;128:545–55:96702. 10.1172/JCI96702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen L, Collij V, Jaeger M, et al. Gut microbial co-abundance networks show specificity in inflammatory bowel disease and obesity. Nat Commun 2020;11:4018. 10.1038/s41467-020-17840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fan Y, Pedersen O. Gut Microbiota in human metabolic health and disease. Nat Rev Microbiol 2021;19:55–71. 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- 75. Tilg H, Zmora N, Adolph TE, et al. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol 2020;20:40–54. 10.1038/s41577-019-0198-4 [DOI] [PubMed] [Google Scholar]
- 76. Kawano Y, Edwards M, Huang Y, et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell 2022;185:3501–19. 10.1016/j.cell.2022.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Suez J, Cohen Y, Valdés-Mas R, et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022;185:3307–28. 10.1016/j.cell.2022.07.016 [DOI] [PubMed] [Google Scholar]
- 78. Do MH, Lee E, Oh M-J, et al. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018;10:761. 10.3390/nu10060761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal Microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 2014;147:1363–77. 10.1053/j.gastro.2014.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Makki K, Brolin H, Petersen N, et al. 6Α-Hydroxylated bile acids mediate Tgr5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut 2023;72:314–24. 10.1136/gutjnl-2021-326541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol 2019;15:226–37. 10.1038/s41574-019-0168-8 [DOI] [PubMed] [Google Scholar]
- 82. Adolph TE, Zhang J. Diet fuelling inflammatory bowel diseases: preclinical and clinical concepts. Gut 2022;71:2574–86. 10.1136/gutjnl-2021-326575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016;65:1812–21. 10.1136/gutjnl-2015-309957 [DOI] [PubMed] [Google Scholar]
- 84. Wu GD, Compher C, Chen EZ, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016;65:63–72. 10.1136/gutjnl-2014-308209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Naimi S, Viennois E, Gewirtz AT, et al. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021;9:66. 10.1186/s40168-020-00996-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017;66:1414–27. 10.1136/gutjnl-2016-313099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zuo T, Ng SC. The gut Microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol 2018;9:2247. 10.3389/fmicb.2018.02247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hosseinkhani F, Heinken A, Thiele I, et al. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes 2021;13:1–22:1882927. 10.1080/19490976.2021.1882927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Verdugo-Meza A, Ye J, Dadlani H, et al. Connecting the dots between inflammatory bowel disease and metabolic syndrome: a focus on gut-derived metabolites. Nutrients 2020;12:1434. 10.3390/nu12051434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Michaudel C, Danne C, Agus A, et al. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut 2023;72:1296–307. 10.1136/gutjnl-2022-327337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host & Microbe 2018;23:716–24. 10.1016/j.chom.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 92. Natividad JM, Agus A, Planchais J, et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab 2018;28:737–49. 10.1016/j.cmet.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 93. Chassaing B, Raja SM, Lewis JD, et al. Colonic microbiota encroachment correlates with dysglycemia in humans. Cell Mol Gastroenterol Hepatol 2017;4:205–21. 10.1016/j.jcmgh.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys Gut 2006;55:426–31. 10.1136/gut.2005.069476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. results from a prospective population-based study. Gut 2008;57:1518–23. 10.1136/gut.2007.146357 [DOI] [PubMed] [Google Scholar]
- 96. Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003;107:391–7. 10.1161/01.cir.0000055014.62083.05 [DOI] [PubMed] [Google Scholar]
- 97. Esposito K, Giugliano D. The metabolic syndrome and inflammation: association or causation? Nutr Metab Cardiovasc Dis 2004;14:228–32. 10.1016/s0939-4753(04)80048-6 [DOI] [PubMed] [Google Scholar]
- 98. Lee YS, Olefsky J. Chronic tissue inflammation and metabolic disease. Genes Dev 2021;35:307–28. 10.1101/gad.346312.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Singh S, Kullo IJ, Pardi DS, et al. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol 2015;12:26–35. 10.1038/nrgastro.2014.202 [DOI] [PubMed] [Google Scholar]
- 100. Wilson PWF, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–72. 10.1161/CIRCULATIONAHA.105.539528 [DOI] [PubMed] [Google Scholar]
- 101. Tune JD, Goodwill AG, Sassoon DJ, et al. Cardiovascular consequences of metabolic syndrome. Transl Res 2017;183:57–70. 10.1016/j.trsl.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004;110:1245–50. 10.1161/01.CIR.0000140677.20606.0E [DOI] [PubMed] [Google Scholar]
- 103. Novacek G, Weltermann A, Sobala A, et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology 2010;139:779–87. 10.1053/j.gastro.2010.05.026 [DOI] [PubMed] [Google Scholar]
- 104. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63. 10.1016/S0140-6736(09)61963-2 [DOI] [PubMed] [Google Scholar]
- 105. Sridhar ARM, Parasa S, Navaneethan U, et al. Comprehensive study of cardiovascular morbidity in hospitalized inflammatory bowel disease patients. J Crohns Colitis 2011;5:287–94. 10.1016/j.crohns.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 106. Conrad N, Verbeke G, Molenberghs G, et al. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 2022;400:733–43. 10.1016/S0140-6736(22)01349-6 [DOI] [PubMed] [Google Scholar]
- 107. Méndez-Sánchez N, Bugianesi E, Gish RG, et al. Global multi-Stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol 2022;7:388–90. 10.1016/S2468-1253(22)00062-0 [DOI] [PubMed] [Google Scholar]
- 108. Jiang K, Chen B, Lou D, et al. Systematic review and meta-analysis: association between obesity/overweight and surgical complications in IBD. Int J Colorectal Dis 2022;37:1485–96. 10.1007/s00384-022-04190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Donini LM, Busetto L, Bischoff SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts 2022;15:321–35. 10.1159/000521241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ding NS, Malietzis G, Lung PFC, et al. The body composition profile is associated with response to anti-TNF therapy in Crohn’s disease and may offer an alternative dosing paradigm. Aliment Pharmacol Ther 2017;46:883–91. 10.1111/apt.14293 [DOI] [PubMed] [Google Scholar]
- 111. Fatani H, Olaru A, Stevenson R, et al. Systematic review of sarcopenia in inflammatory bowel disease. Clin Nutr 2023;42:1276–91. 10.1016/j.clnu.2023.05.002 [DOI] [PubMed] [Google Scholar]
- 112. Adams DW, Gurwara S, Silver HJ, et al. Sarcopenia is common in overweight patients with inflammatory bowel disease and may predict need for surgery. Inflamm Bowel Dis 2017;23:1182–6. 10.1097/MIB.0000000000001128 [DOI] [PubMed] [Google Scholar]
- 113. Bischoff SC, Escher J, Hébuterne X, et al. ESPEN practical guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr 2020;39:632–53. 10.1016/j.clnu.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 114. Bischoff SC, Ockenga J, Eshraghian A, et al. Practical guideline on obesity care in patients with gastrointestinal and liver diseases - joint ESPEN/UEG guideline. Clinical Nutrition 2023;42:987–1024. 10.1016/j.clnu.2023.03.021 [DOI] [PubMed] [Google Scholar]
- 115. Karczewski J, Begier-Krasińska B, Staszewski R, et al. Obesity and the risk of gastrointestinal cancers. Dig Dis Sci 2019;64:2740–9. 10.1007/s10620-019-05603-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yu J, Refsum E, Perrin V, et al. Inflammatory bowel disease and risk of adenocarcinoma and neuroendocrine tumors in the small bowel. Ann Oncol 2022;33:649–56. 10.1016/j.annonc.2022.02.226 [DOI] [PubMed] [Google Scholar]
- 117. Huang J, Chan SC, Fung YC, et al. Incidence, risk factors, and temporal trends of small intestinal cancer: A global analysis of cancer registries. Gastroenterology 2023;165:600–12. 10.1053/j.gastro.2023.05.043 [DOI] [PubMed] [Google Scholar]
- 118. Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS ONE 2013;8:e53916. 10.1371/journal.pone.0053916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wunderlich CM, Ackermann PJ, Ostermann AL, et al. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage Polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat Commun 2018;9:1646. 10.1038/s41467-018-03773-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tsounis EP, Aggeletopoulou I, Mouzaki A, et al. Creeping fat in the pathogenesis of Crohn’s disease: an orchestrator or a silent bystander Inflamm Bowel Dis 2023;29:1826–36. 10.1093/ibd/izad095 [DOI] [PubMed] [Google Scholar]
- 121. Avgerinos KI, Spyrou N, Mantzoros CS, et al. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism 2019;92:121–35. 10.1016/j.metabol.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 122. Rubin DT, Huo D, Kinnucan JA, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol 2013;11:1601–8. 10.1016/j.cgh.2013.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shah SC, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology 2022;162:715–30. 10.1053/j.gastro.2021.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Viganò C, Palermo A, Mulinacci G, et al. Prevalence of disease-related malnutrition and micronutrients deficit in patients with inflammatory bowel disease: a multicentric cross-sectional study by the GSMII (inflammatory bowel disease study group). Inflamm Bowel Dis 2023. 10.1093/ibd/izad146 [DOI] [PubMed] [Google Scholar]
- 125. Ogawa M, Okamura M, Inoue T, et al. Relationship between nutritional status and clinical outcomes among older individuals using long-term care services: a systematic review and meta-analysis. Clinical Nutrition ESPEN 2024;59:365–77. 10.1016/j.clnesp.2023.11.024 [DOI] [PubMed] [Google Scholar]
- 126. Massironi S, Viganò C, Palermo A, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2023;8:579–90. 10.1016/S2468-1253(23)00011-0 [DOI] [PubMed] [Google Scholar]
- 127. Bischoff SC, Bager P, Escher J, et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. Clinical Nutrition 2023;42:352–79. 10.1016/j.clnu.2022.12.004 [DOI] [PubMed] [Google Scholar]
- 128. Kim S-R, Kim H-N, Song S-W. Associations between mental health, quality of life, and obesity/metabolic risk phenotypes. Metabolic Syndrome and Related Disorders 2020;18:347–52. 10.1089/met.2020.0028 [DOI] [PubMed] [Google Scholar]
- 129. Saboya PP, Bodanese LC, Zimmermann PR, et al. Metabolic syndrome and quality of life: a systematic review. Rev Lat Am Enfermagem 2016;24:e2848. 10.1590/1518-8345.1573.2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev 2001;2:219–29. 10.1046/j.1467-789x.2001.00040.x [DOI] [PubMed] [Google Scholar]
- 131. Knowles SR, Keefer L, Wilding H, et al. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part II. Inflamm Bowel Dis 2018;24:966–76. 10.1093/ibd/izy015 [DOI] [PubMed] [Google Scholar]
- 132. Knowles SR, Graff LA, Wilding H, et al. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part I. Inflamm Bowel Dis 2018;24:742–51. 10.1093/ibd/izx100 [DOI] [PubMed] [Google Scholar]
- 133. Khakoo NS, Ioannou S, Khakoo NS, et al. Impact of obesity on inflammatory bowel disease. Curr Gastroenterol Rep 2022;24:26–36. 10.1007/s11894-022-00840-x [DOI] [PubMed] [Google Scholar]
- 134. Rasheed S, Woods RT. Malnutrition and quality of life in older people: a systematic review and meta-analysis. Ageing Research Reviews 2013;12:561–6. 10.1016/j.arr.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 135. Armuzzi A, Liguori G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: a narrative review. Digestive and Liver Disease 2021;53:803–8. 10.1016/j.dld.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 136. Bassi M, Singh S. Impact of obesity on response to biologic therapies in patients with inflammatory bowel diseases. BioDrugs 2022;36:197–203. 10.1007/s40259-022-00522-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yarur AJ, Bruss A, Moosreiner A, et al. Higher intra-abdominal visceral adipose tissue mass is associated with lower rates of clinical and endoscopic remission in patients with inflammatory bowel diseases initiating biologic therapy: results of the constellation study. Gastroenterology 2023;165:963–75. 10.1053/j.gastro.2023.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sodhi M, Rezaeianzadeh R, Kezouh A, et al. Risk of gastrointestinal adverse events associated with glucagon-like Peptide-1 receptor agonists for weight loss. JAMA 2023;330:1795–7. 10.1001/jama.2023.19574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts 2015;8:402–24. 10.1159/000442721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bray GA, Ryan DH. Medical therapy for the patient with obesity. Circulation 2012;125:1695–703. 10.1161/CIRCULATIONAHA.111.026567 [DOI] [PubMed] [Google Scholar]
- 141. Wood GC, Bailey-Davis L, Benotti P, et al. Effects of sustained weight loss on outcomes associated with obesity Comorbidities and Healthcare resource utilization. PLoS One 2021;16:e0258545. 10.1371/journal.pone.0258545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tsigos C, Hainer V, Basdevant A, et al. Management of obesity in adults: European clinical practice guidelines. Obes Facts 2008;1:106–16. 10.1159/000126822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Durrer Schutz D, Busetto L, Dicker D, et al. European practical and patient-centred guidelines for adult obesity management in primary care. Obes Facts 2019;12:40–66. 10.1159/000496183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mabeza RM, Vadlakonda A, Chervu N, et al. Short-term outcomes of bariatric surgery in patients with inflammatory bowel disease: a national analysis. Surgery for Obesity and Related Diseases 2024;20:146–52. 10.1016/j.soard.2023.10.006 [DOI] [PubMed] [Google Scholar]
- 145. Mian A, Khan S. Systematic review: outcomes of Bariatric surgery in patients with inflammatory bowel disease and de-Novo IBD development after bariatric surgery. Surgeon 2023;21:e71–7. 10.1016/j.surge.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 146. Wilson NC, Dilsaver DB, Walters RW, et al. Bariatric surgery outcomes in patients with inflammatory bowel disease in the United States: an analysis of the nationwide readmissions database. OBES SURG 2024. 10.1007/s11695-024-07111-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lin IC, Liu H. Impact of bariatric surgery on outcomes of patients with inflammatory bowel disease: a nationwide inpatient sample analysis, 2005-2018. OBES SURG 2024;34:479–86. 10.1007/s11695-023-07023-1 [DOI] [PubMed] [Google Scholar]
- 148. Kedia S, Virmani S, K Vuyyuru S, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut 2022;71:2401–13. 10.1136/gutjnl-2022-327811 [DOI] [PubMed] [Google Scholar]
- 149. Qiu P, Ishimoto T, Fu L, et al. The gut microbiota in inflammatory bowel disease. Front Cell Infect Microbiol 2022;12:733992. 10.3389/fcimb.2022.733992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. de Groot PF, Frissen MN, de Clercq NC, et al. Fecal microbiota transplantation in metabolic syndrome: history, present and future. Gut Microbes 2017;8:253–67. 10.1080/19490976.2017.1293224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab 2017;26:611–9. 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 152. Jacobs JP, Goudarzi M, Lagishetty V, et al. Crohn’s disease in endoscopic remission, obesity, and cases of high genetic risk demonstrates overlapping shifts in the colonic mucosal-luminal interface microbiome. Genome Med 2022;14:91. 10.1186/s13073-022-01099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Britton GJ, Contijoch EJ, Mogno I, et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt(+). Regulatory T Cells and Exacerbate Colitis in Mice Immunity 2019;50:212–24. 10.1016/j.immuni.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhao HL, Chen SZ, Xu HM, et al. Efficacy and safety of fecal Microbiota transplantation for treating patients with ulcerative colitis: a systematic review and meta-analysis. J Dig Dis 2020;21:534–48. 10.1111/1751-2980.12933 [DOI] [PubMed] [Google Scholar]
- 155. Yu X-H, Cao R-R, Yang Y-Q, et al. Identification of causal metabolites related to multiple autoimmune diseases. Hum Mol Genet 2022;31:604–13. 10.1093/hmg/ddab273 [DOI] [PubMed] [Google Scholar]
- 156. Di’Narzo AF, Houten SM, Kosoy R, et al. Integrative analysis of the inflammatory bowel disease serum Metabolome improves our understanding of genetic etiology and points to novel putative therapeutic targets. Gastroenterology 2022;162:828–43. 10.1053/j.gastro.2021.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Dragasevic S, Stankovic B, Kotur N, et al. Metabolic syndrome in inflammatory bowel disease: association with genetic markers of obesity and inflammation. Metabolic Syndrome and Related Disorders 2020;18:31–8. 10.1089/met.2019.0090 [DOI] [PubMed] [Google Scholar]
- 158. Handley D, Rafey MF, Almansoori S, et al. Higher waist hip ratio genetic risk score is associated with reduced weight loss in patients with severe obesity completing a meal replacement programme. J Pers Med 2022;12:1881. 10.3390/jpm12111881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. The Lancet 2022;399:2015–30. 10.1016/S0140-6736(22)00467-6 [DOI] [PubMed] [Google Scholar]
- 160. D’Haens G, Dubinsky M, Kobayashi T, et al. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2023;388:2444–55. 10.1056/NEJMoa2207940 [DOI] [PubMed] [Google Scholar]
- 161. Martins LMS, Perez MM, Pereira CA, et al. Interleukin-23 promotes intestinal T helper Type17 immunity and ameliorates obesity-associated metabolic syndrome in a murine high-fat diet model. Immunology 2018;154:624–36. 10.1111/imm.12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med 2000;343:1467–76. 10.1056/NEJM200011163432007 [DOI] [PubMed] [Google Scholar]
- 163. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017;542:177–85. 10.1038/nature21363 [DOI] [PubMed] [Google Scholar]
- 164. Donath MY, Dinarello CA, Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat Rev Immunol 2019;19:734–46. 10.1038/s41577-019-0213-9 [DOI] [PubMed] [Google Scholar]
- 165. Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–26. 10.1056/NEJMoa065213 [DOI] [PubMed] [Google Scholar]
- 166. Miranda-Filloy JA, Llorca J, Carnero-López B, et al. TNF-alpha antagonist therapy improves insulin sensitivity in non-diabetic ankylosing spondylitis patients. Clin Exp Rheumatol 2012;30:850–5. [PubMed] [Google Scholar]
- 167. Pina T, Armesto S, Lopez‐Mejias R, et al. Anti-TNF-Α therapy improves insulin sensitivity in non-diabetic patients with psoriasis: a 6-month prospective study . Acad Dermatol Venereol 2015;29:1325–30. 10.1111/jdv.12814 [DOI] [PubMed] [Google Scholar]
- 168. Sethi JK, Hotamisligil GS. Metabolic messengers: tumour necrosis factor. Nat Metab 2021;3:1302–12. 10.1038/s42255-021-00470-z [DOI] [PubMed] [Google Scholar]
- 169. U.S. Department of Agriculture and U.S. Department of Health and Human Services . Dietary Guidelines for Americans, 2020-2025. 9th edn. DietaryGuidelines.gov, 2020. [Google Scholar]
- 170. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–28. 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- 171. Fahed G, Aoun L, Bou Zerdan M, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci 2022;23:786. 10.3390/ijms23020786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol 2018;15:21–32. 10.1038/s41574-018-0096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Shimobayashi M, Albert V, Woelnerhanssen B, et al. Insulin resistance causes inflammation in Adipose tissue. J Clin Invest 2018;128:1538–50:96139. 10.1172/JCI96139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol 2016;12:15–28. 10.1038/nrendo.2015.189 [DOI] [PubMed] [Google Scholar]
- 175. Matsuzawa Y, Funahashi T, Kihara S, et al. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 2004;24:29–33. 10.1161/01.ATV.0000099786.99623.EF [DOI] [PubMed] [Google Scholar]
- 176. Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 2012;142:711–25. 10.1053/j.gastro.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 177. Parikh K, Antanaviciute A, Fawkner-Corbett D, et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019;567:49–55. 10.1038/s41586-019-0992-y [DOI] [PubMed] [Google Scholar]
- 178. Friedrich M, Pohin M, Jackson MA, et al. IL-1-driven Stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat Med 2021;27:1970–81. 10.1038/s41591-021-01520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kong L, Pokatayev V, Lefkovith A, et al. The landscape of immune dysregulation in crohn’s disease revealed through single-cell transcriptomic profiling in the ileum and colon. Immunity 2023;56:444–58. 10.1016/j.immuni.2023.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Makki K, Deehan EC, Walter J, et al. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018;23:705–15. 10.1016/j.chom.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 181. Munukka E, Rintala A, Toivonen R, et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat Fed mice. ISME J 2017;11:1667–79. 10.1038/ismej.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Martín R, Chain F, Miquel S, et al. The commensal bacterium faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflammatory Bowel Diseases 2014;20:417–30. 10.1097/01.MIB.0000440815.76627.64 [DOI] [PubMed] [Google Scholar]
- 183. Sarkar A, Mitra P, Lahiri A, et al. Butyrate limits inflammatory macrophage niche in NASH. Cell Death Dis 2023;14:332. 10.1038/s41419-023-05853-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Zheng L, Kelly CJ, Battista KD, et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of Claudin-2. J Immunol 2017;199:2976–84. 10.4049/jimmunol.1700105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sun M, Wu W, Chen L, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun 2018;9:3555. 10.1038/s41467-018-05901-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Li J, Lin S, Vanhoutte PM, et al. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in apoe-/- mice. Circulation 2016;133:2434–46. 10.1161/CIRCULATIONAHA.115.019645 [DOI] [PubMed] [Google Scholar]
- 187. Wang L, Tang L, Feng Y, et al. A purified membrane protein from akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of Cd8(+) T cells in mice . Gut 2020;69:1988–97. 10.1136/gutjnl-2019-320105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92–6. 10.1038/nature14232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012;487:104–8. 10.1038/nature11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Caesar R, Tremaroli V, Kovatcheva-Datchary P, et al. Crosstalk between gut Microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 2015;22:658–68. 10.1016/j.cmet.2015.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]