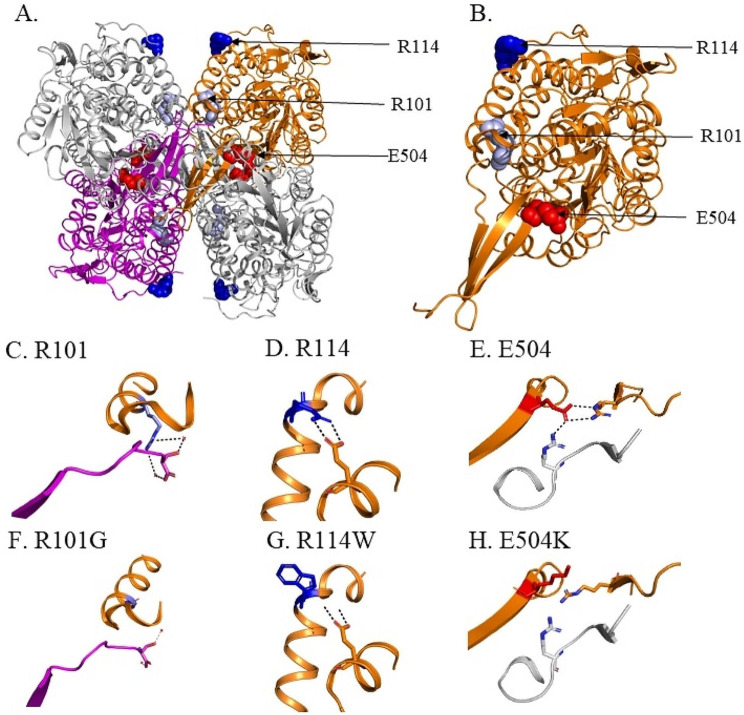
Fig. 4.
Location of R101G R114W and E504K variants in ALDH2 and impact of these variants. (A) Crystal structure of ALDH2, with 3 amino acids within the tetramer highlighted (R101 (light blue), R114 (dark blue) and E504 (red)). (B) Location of R101 (light blue), R114 (dark blue) and E504 (red) in the ALDH2 subunit. H-bond interactions between (C) R101 with S517 of another subunit via conserved water. (D) R114 with E227 of the same subunit. (E) E504K with R281 of one subunit and R492 of another ALDH2 subunit. (F) The R101G variant leads to loss of H-bonds with S517 of the opposite subunit. (G) R114W variant leads to loss of H-bonds with E227 within the same subunit and (H) E504K leads to loss of H-bonds with R281 within the same subunit and R492 of another subunit at the dimer interface