Abstract
OBJECTIVE: To compare in vitro percutaneous absorption of methyl-parathion dissolved in an acetone vehicle and in the form of a commercial formulation. METHODS: Penetration through the human skin was measured in Franz diffusion cells with full thickness skin from a human cadaver as the membrane. The two tailed non-parametric Mann-Whitney U test was used to compare the cumulative diffusion of methyl-parathion in the receptor fluid of the cells at various time intervals. RESULTS: In vitro skin penetration of methyl-parathion was significantly higher with the commercial formulation. The percentage of the applied dose absorbed after 24 hours was 5.20% v 1.35%. The mean lag time was < 8 hours. CONCLUSION: Assessments of uptake and internal dose after exposure to pesticides should be based on the commercial products rather than active ingredients, because of the crucial role of the vehicle, as shown in this study.
Full text
PDF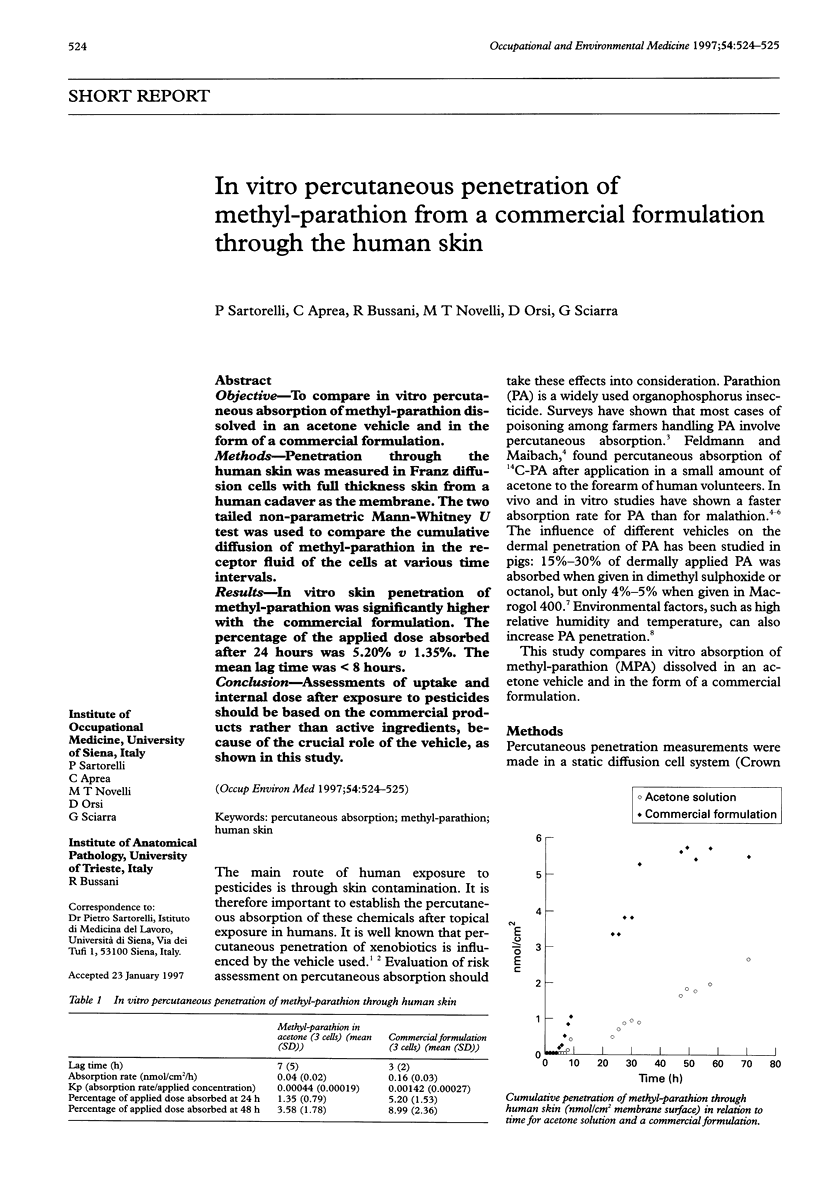

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronaugh R. L., Franz T. J. Vehicle effects on percutaneous absorption: in vivo and in vitro comparisons with human skin. Br J Dermatol. 1986 Jul;115(1):1–11. doi: 10.1111/j.1365-2133.1986.tb06214.x. [DOI] [PubMed] [Google Scholar]
- Chang S. K., Riviere J. E. Percutaneous absorption of parathion in vitro in porcine skin: effects of dose, temperature, humidity, and perfusate composition on absorptive flux. Fundam Appl Toxicol. 1991 Oct;17(3):494–504. doi: 10.1016/0272-0590(91)90200-n. [DOI] [PubMed] [Google Scholar]
- Chang S. K., Williams P. L., Dauterman W. C., Riviere J. E. Percutaneous absorption, dermatopharmacokinetics and related bio-transformation studies of carbaryl, lindane, malathion, and parathion in isolated perfused porcine skin. Toxicology. 1994 Aug 12;91(3):269–280. doi: 10.1016/0300-483x(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Feldmann R. J., Maibach H. I. Percutaneous penetration of some pesticides and herbicides in man. Toxicol Appl Pharmacol. 1974 Apr;28(1):126–132. doi: 10.1016/0041-008x(74)90137-9. [DOI] [PubMed] [Google Scholar]
- Franz T. J. Percutaneous absorption on the relevance of in vitro data. J Invest Dermatol. 1975 Mar;64(3):190–195. doi: 10.1111/1523-1747.ep12533356. [DOI] [PubMed] [Google Scholar]
- Shah P. V., Monroe R. J., Guthrie F. E. Comparative rates of dermal penetration of insecticides in mice. Toxicol Appl Pharmacol. 1981 Jul;59(3):414–423. doi: 10.1016/0041-008x(81)90293-3. [DOI] [PubMed] [Google Scholar]
- Shih J. H., Wu Z. Q., Wang Y. L., Zhang Y. X., Xue S. Z., Gu X. Q. Prevention of acute parathion and demeton poisoning in farmers around Shanghai. Scand J Work Environ Health. 1985;11 (Suppl 4):49–54. [PubMed] [Google Scholar]


