Abstract
Carbohydrates and glycoproteins modulate key biological functions. However, experimental structure determination of sugar polymers is notoriously difficult. Computational approaches can aid in carbohydrate structure prediction, structure determination, and design. In this work, we developed a glycan-modeling algorithm, GlycanTreeModeler, that computationally builds glycans layer-by-layer, using adaptive kernel density estimates (KDE) of common glycan conformations derived from data in the Protein Data Bank (PDB) and from quantum mechanics (QM) calculations. GlycanTreeModeler was benchmarked on a test set of glycan structures of varying lengths, or “trees”. Structures predicted by GlycanTreeModeler agreed with native structures at high accuracy for both de novo modeling and experimental density-guided building. We employed these tools to design de novo glycan trees into a protein nanoparticle vaccine to shield regions of the scaffold from antibody recognition, and experimentally verified shielding. This work will inform glycoprotein model prediction, glycan masking, and further aid computational methods in experimental structure determination and refinement.
Author summary
Many biological proteins are chemically modified to induce specific structure and function. Carbohydrates (glycans) are one such modification that play an important role in signaling, stability, solubility, aggregation, and the immune system. In this work, we have developed and extensively benchmarked a computational protocol for predicting the structures of these glycans, while improving the Rosetta Software Suite through the development of new general analysis frameworks, such as the SimpleMetric system and extensive glycan tools for modeling and design. We describe the benchmarking, optimization, and use of a novel computational method for three dimensional modeling of glycans and glyco-conjugates called the GlycanTreeModeler. This method is unique in that it grows glycans layer-by-layer using extensive data-driven methods. We thoroughly benchmark the method and detail iterative improvement of the GlycanTreeModeler through scoring and kinematic improvements, and show how these methods can be useful in glycan-related computational tasks and glycan masking of a novel vaccine scaffold in-vitro and in-vivo.
Introduction
Carbohydrates and glycoproteins are ubiquitous in biological organisms [1]. Viral glycoproteins such as HIV envelope trimer, influenza hemagglutinin, and SARS-CoV-2 spike, employ N-linked glycosylation as an immune evasion strategy, taking advantage of the fact that host glycans on the surface of proteins are usually recognized as “self” by the adaptive immune system [2]. Yet, HIV broadly neutralizing antibodies often target glycans as part of their epitopes [3] [4] [5]. Small carbohydrate residues attached to serine or threonine can act in signaling pathways akin to phosphorylation [6], while glycans on the constant region of antibodies act as mediators of effector function [7] [8]. Glycans can also improve stability [9] and solubility [10], reduce aggregation [11], and even improve biological drug-targeting and vaccine design through glycan masking of off-target regions [12]. However, in the context of a series of protein nanoparticle immunogens, we recently discovered that glycan masking of the protein nanoparticle scaffold itself is unlikely to enhance antigen-specific antibody responses, especially when the displayed antigen is immunodominant over the nanoparticle scaffold [13]. But we also recently showed that high-density and high-mannose glycans on protein nanoparticle surfaces increase lymph node trafficking and antibody responses against the nanoparticle in a density- and mannose-dependent manner [14]. Thus, optimizing the density and composition of glycans displayed on protein-based vaccines—either on the antigen and/or protein nanoparticle scaffold—provides a framework for engineering glycan recognition to optimize vaccine efficacy.
The biosynthesis of glycoconjugates is complex. Carbohydrates can be attached to certain amino acid residues including serine, threonine, asparagine, and (rarely) tryptophan through covalent modification, forming glycoproteins. The attachment can be made to nitrogen, oxygen, or carbon atoms, (known as N-, O-, or C-linked glycosylation, respectively), with each process involving a multitude of enzymes, sugar moieties and resulting carbohydrate structures. These processes are stochastic in nature, producing glycoproteins that are heterogeneous in both the occupancy of a glycan at the glycosylation site (macro-heterogenicity) and the chemical makeup of the N-, C-, or O-linked glycan (micro-heterogenicity) [15].
The most common form of glycosylation observed in glycoprotein structures is N-linked glycosylation. Initiation of this process occurs during translation, by the protein oligosaccharyltransferase (OST), which recognizes a multi-residue consensus motif, or sequon, of NX(S/T) (where X is any residue except proline), and covalently attaches a lipid-linked core-oligosaccharide to the asparagine residue through an N-glycoside linkage 1. This process is not deterministic (not every sequon results in attachment of a glycan) and certain amino acids in and around the sequon motif can affect the efficiency of this process, resulting in higher or lower glycan occupancy at the site [16] [17].
Upon successful protein folding in the endoplasmic reticulum, the initial N-linked glycan is “trimmed down” by removal of several terminal glucosyl residues, while many sugar processing enzymes in the Golgi apparatus can add or remove sugar residues from the nascent branched sugar (tree). The resulting chemical makeup of the glycan tree depends on which enzymes are available in the Golgi, which is heavily influenced by species, disease state [18], developmental stage [19]; and the local structure, sequence, and environment of the glycosylation site [20]. In addition, a particular glycosylation site can result in vastly different glycans [21], though this can be controlled to some extent through various bioengineering techniques [15] [22] [23].
Glycans are also conformationally flexible, being highly hydrophilic and typically exposed on the surface of proteins, with a large number of conformational degrees of freedom. However, as has been observed in molecular dynamics and NMR experiments, glycan conformations can be influenced by their structural environment [24]. Through the plethora of high-resolution crystallographic and cryo-EM studies, we also know that glycans can adopt stable conformations with well-defined density observed for many of the glycan residues in each tree, especially towards the root of the glycan tree, even for some unrestrained glycans [25] [26],. Presumably, these low-energy, stable conformations are occupied at higher frequency in solution. In addition, a recent QM study on glycan torsional energies showed that the QM-derived conformational preferences of glycan torsions match well with glycan structures analyzed from the protein data bank, indicating that conformational diversity is also influenced by the chemical makeup of each glycan structure [27].
Given the complex chemistry and conformational diversity involved, accurate modeling of glycans is currently a grand challenge in computational biology. Computational glycobiology tools and webapps have been developed for protein glycosylations [28], validation of carbohydrate structural chemistry [29], statistical analysis [30], and docking [31] [32], Common methods in glycoprotein modeling typically involve molecular dynamics (MD) simulations [33] or adding glycans by manual placement and conformational tweaking into their density for structure determination [34]. Recently, a new method for automatic building of glycan structures from sequence was described [35]; this method, the CHARM-GUI Glycan Modeler, was benchmarked only up to the first and second sugar.
Here we describe a new glycan modeling algorithm built within the Rosetta software suite, a platform that incorporates state-of-the-art applications and modules for a variety of macromolecular modeling and design tasks [36]. The new algorithm provides user interfaces for the creation of tailor-made protocols [37] [38], and includes a reliable knowledge-based energy function to evaluate models and designs [39]. We build on earlier work that enabled representing and evaluating carbohydrate structures within Rosetta [40] and in loading, representing, and refining glycans from the Protein Data Bank [41]. We expand on this foundational work through the addition of new carbohydrate-specific sampling methods, an updated conformer database employing adaptive kernel density estimates, a new framework for general analysis in Rosetta (SimpleMetrics), and a new algorithm for accurately modeling complex carbohydrates, the GlycanTreeModeler.
We rigorously benchmark the new method on a set of diverse high-resolution crystal structures of glycans in symmetric crystal environments using the new analysis framework SimpleMetrics and a new application called rosetta_scripts_jd3, and we show that the GlycanTreeModeler is capable of recapitulating native glycan structures with high accuracy both through de novo and density-guided modeling [42]. We then applied our glycan modeling protocol with Rosetta sequence design of glycan sequons to engineer optimal new glycans onto a protein nanoparticle vaccine scaffold and evaluated changes in immune responses. We observed reduced reactivity to the underlying protein surface in immunization experiments, thus demonstrating that glycans can be computationally engineered to tailor immunogenicity of vaccines.
Results
Benchmarking tools
In order to examine the performance of GlycanTreeModeler, we built a new benchmarking infrastructure in Rosetta. We developed the SimpleMetrics framework within the XML interface to Rosetta (RosettaScripts [37]), which allows for robust analysis through more than 20 associated structural and energetic metrics, with data reporting at any step in a RosettaScripts protocol. To facilitate large scale benchmarking, we developed a general application for parallel RosettaScripts computing, rosetta_scripts_jd3, enabling glycan calculations to be run in parallel on a high-performance computing cluster. This application can run multiple jobs within a single parallel run of Rosetta, with individually configured glycan trees to be modeled, and any associated input files for each. The SimpleMetric framework and rosetta_scripts_jd3 application are reviewed in detail in S1 Text.
Glycan structure set
The Rosetta GlycanTreeModeler algorithm was benchmarked against a set of 25 unique N-linked glycan trees in their crystal arrangement ranging from three to twelve residues, across 19 unrelated glycoprotein structures of better than 2 Å resolution, totaling 139 sugar residues. Each glycan tree was checked for chemical and structural inconsistencies (such as incorrect isoform assignments, wrong linkages, or missing atoms) using the glycosciences.de pdb-care webserver (which filtered many of our initial glycan list) [29]. It should also be noted that some of the structures are likely substructures of larger glycans. Preparation and analysis of the structures can be found in S1 Text.
De novo modeling
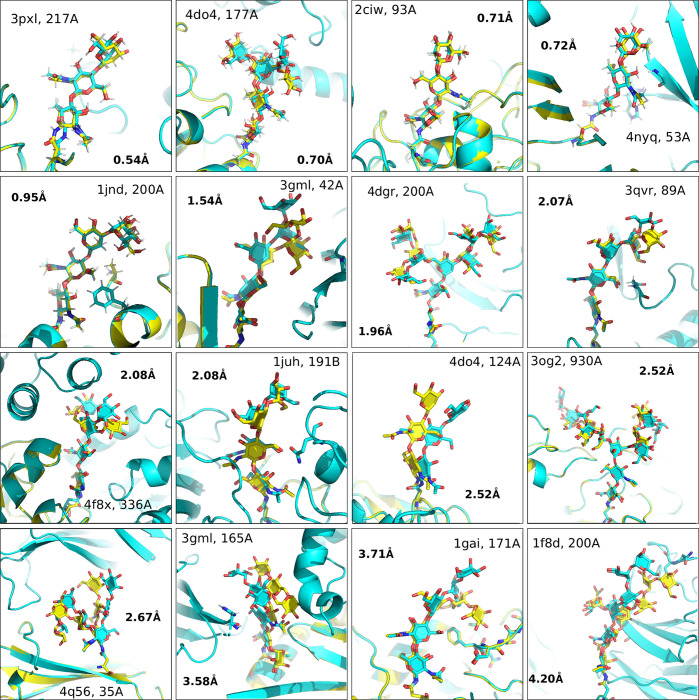
Using the optimized protocol and scoring function found during protocol optimization (see methods), benchmarking was done on the set of 25 glycans described above. Across the benchmark dataset, the median RMSD of the glycan predictions to the native structures was 2.7 Å, while the mean was 5 Å. For the first two residues of the glycan tree, the median was 1.28 Å with a mean of 2.17 Å. Of the 25 glycan trees, 20% of the glycans were predicted at < 1 Å accuracy and 72% (18/25) of the glycans were predicted at < 5 Å accuracy (Figs 1 and 2). The largest glycan in our dataset, with twelve residues, was benchmarked at 2.5 Å. Full results for each glycan are listed in S1 Table.
Fig 1. Near native structures from de novo modeling.
(Top Scoring models for each glycan in the benchmark set) Yellow = Native, Cyan = Model.
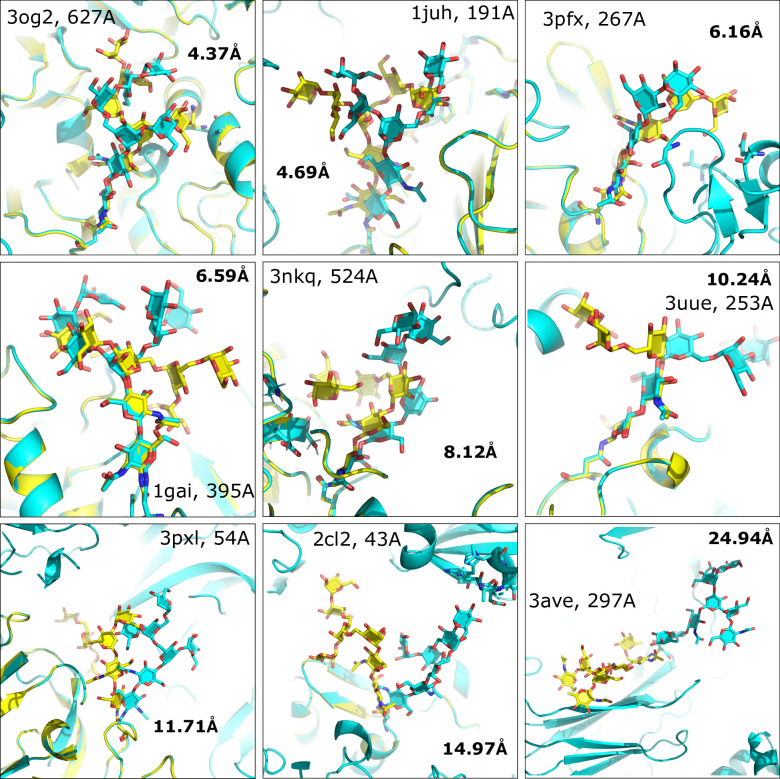
Fig 2. De novo predictions, farthest from native.
(Top Scoring models for each glycan in the benchmark set) Yellow = Native, Cyan = Model
It is also useful to understand how well the algorithm predicts the internal structure of the glycans, as a single dihedral angle change at the root of the glycan can significantly change the overall structure of the glycan relative to the protein. For each of these structures, the same lowest-energy models were superimposed onto the input glycan. The median superimposed RMSD is 1.1 Å, with a mean of 2.7 Å. Overall, 32% (8/25) were < 1 Å RMSD, 64% < 2.5 Å RMSD and 92% of the predictions < 5 Å. Both RMSD measurements of the glycans were generally correlated to each other (S1 Fig).
In addition, most of the glycan benchmarks in our dataset had convergent score vs. RMSD (funnel) plots (S2 Fig). This funnel-like quality is directly related to the ability of the scoring function to discriminate near-native models from decoys and was quantified using the PNear metric [43] that estimates the Boltzmann-weighted probability of finding a system near its native state at various near-native cutoffs (lambdas) (S1 Text). A PNear closer to 1.0 indicates the highest quality funnel possible. The worst-performing glycans in our benchmark set had poor score vs. RMSD funnels, indicating that the scoring function was not able to capture important biophysical properties of the structure (S3 Fig). The worst-performing glycan from the Fc antibody fragment of 3ave, had an RMSD of almost 25 Å with an internal (superimposed) RMSD of 3.6 Å. In this lowest-scoring model (and others), the modeled glycan interacts with the more hydrophilic surface of a crystallographic symmetry mate rather than the more hydrophobic glycan-interacting surface of the parent protein that includes two aromatic rings (S4 Fig). This result is further detailed through the low pNear metrics of the funnel plot with all lambdas being less than .01, showing that the current energy function is unable to score these types of interactions well. However, a scoreterm that accurately represents glycan-aromatic CH-π interactions [44] may improve these results.
Solvent is implicitly represented in most Rosetta applications, but we observe that half of the benchmark glycans have significant crystallographic waters in contact with the surrounding protein. Attempting to understand the effect of waters, we modeled the worst-performing and best-performing glycans and then predicted explicit waters around the glycan for each output decoy using Rosetta-ECO [45] in order to score more native-like conformations that have these bridged waters. However, decoy discrimination as measured by pNear was significantly worse for all lambda cutoffs (even for the best-performing glycans), indicating that even with explicit waters and sufficient near-native sampling distributions, the Rosetta energy function was unable to use this information to accurately distinguish near-native decoys. (S2 Table).
In the benchmark set, the internal (superimposed) RMSDs are generally low in comparison to the overall RMSD (84% < 3 Å), showing that the energy function, guided by the QM-derived sugar_bb energy term, can accurately predict many glycan structures, but may need to be further improved to more accurately score glycan-protein interactions in the future.
Density building
There are an increased number of glycoprotein structures being determined. To assist structure determination, many recent glycan modeling tools have focused on their ability to aid in glycan structure building and refinement using the experimental density, especially for structures with many resolved glycans such as HIV Env. We tested the ability of the GlycanTreeModeler to build glycan structures using crystallographic density information to guide modeling and decoy discrimination using integrated density scoring [42]. The experiment was conducted in the same manner as de novo modeling, by first randomizing all backbone dihedral angles of the glycan to be modeled for each output decoy and removing all crystallographic waters. For each of the 25 glycans, the lowest-energy model was used for assessment.
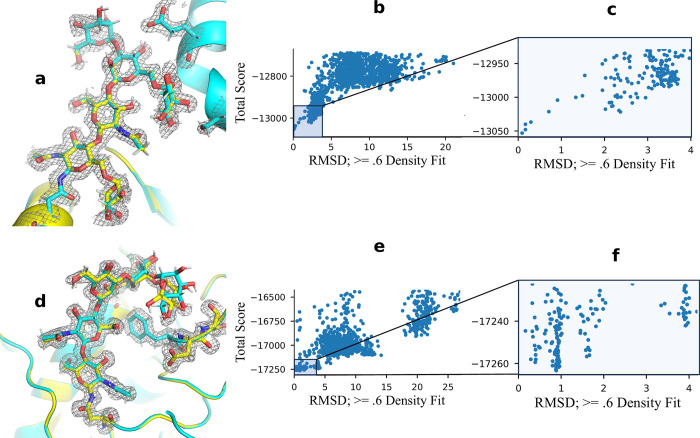
Without further refinement or any additional changes to the protocol, all glycans were modeled at sub-angstrom accuracy. The best glycan in the current benchmark, with six residues, was built at 0.08 Å RMSD to native (3gml position 165A glycan), while the worst, a five-residue glycan, was modeled at 0.88 Å RMSD (1gai position 171A glycan). For both of these glycans, funnel plots were generally good, with respective PNear values of 0.99 and 0.46 at a lambda of 1.0 Å (Fig 3). For 1gai glycan 171A, the last residue in the glycan is twisted in the best model compared to the native and fits two constituent oxygens into the low residue density at a different angle than the solved structure. This twist can clearly be seen in the funnel plot where the distribution of models less than 1 Å is bimodal, indicating two primary close solutions of the electron density. (Fig 3F).
Fig 3.
Best and worst results from density-guided modeling: a. Structural comparison of 3gml 165A glycan; 0.08Å RMSD; cyan = model | yellow = native b (and e). RMSD vs. Score (funnel) plot, top 80% by energy. c (and f). Funnel plot of top 10% models with pNear metrics. d. Structural comparison of 1gai 171A glycan; 0.88Å RMSD; cyan = model | yellow = native
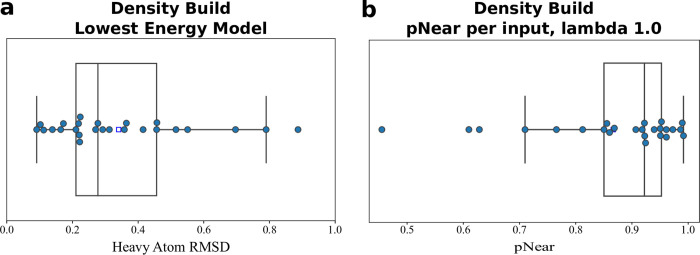
Overall, the GlycanTreeModeler achieved a mean heavy atom RMSD of 0.48 Å using all residues and 0.34 Å using residues that had acceptable fits into the density (133/139 total glycan residues, S1 Text). For both inclusion types, the median RMSD was 0.31 Å and 0.28 Å respectively, while the mean RMSD of the glycan root (first two sugar residues) was .23 Å (Fig 4A) (S3 Table). Values for PNear with lambda of 1.0 Å were generally quite favorable, indicating high-quality funnels, with a mean of 0.86 and median of 0.92 (Fig 4B). These results show that the GlycanTreeModeler can be effective for modeling known glycans into electron density, especially with existing methods refinement [41].
Fig 4.
Density-guided modeling quality: a. Boxplot of the RMSD to native of the best-scoring decoy for each of the benchmarked input glycans. b. Boxplot of the funnel quality for each of the benchmark glycans as measured by the pNear metric. A value closer to 1.0 indicates a high-quality funnel.
Sugar coating protein surfaces
The addition of glycans to exposed protein surfaces can reduce B cell receptor access to underlying surface epitopes; this approach (called “glycan masking”) has been used to decrease the amount of antibodies elicited against off-target epitopes of designed immunogens [12] [46] [47] [48]. Given the predictive capability of the GlycanTreeModeler to model the spatial arrangement of complex glycans, we used the algorithm in combination with RosettaScript SugarCoating methods for sequon design and computational glycosylation to iteratively design four N-linked glycans onto the outer surface of the I53-50A trimeric component of the I53-50 protein nanoparticle scaffold (Fig 5A; details of the design approach are described in Materials and Methods in S1 Text. Designed sequences and designed glycan positions are given in S4 Table). I53-50 was selected as a model immunogen because it is currently in clinical trials as the nanoparticle scaffold for SARS-CoV-2 [49] and RSV [50] vaccines.
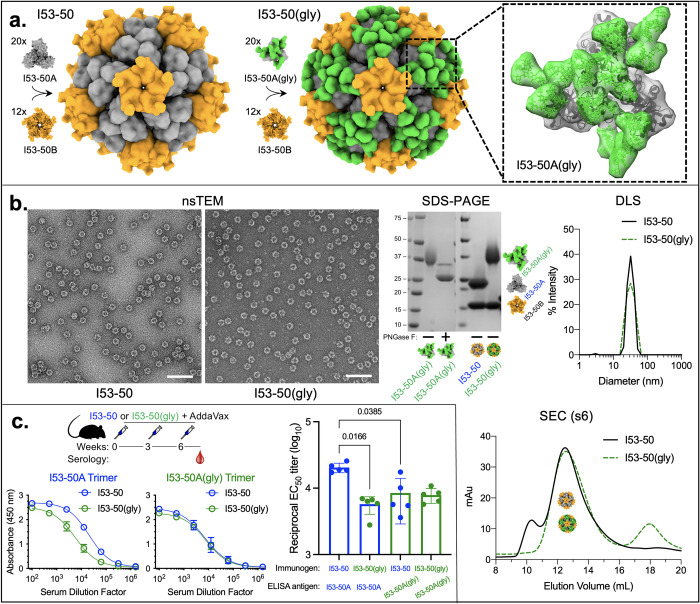
Fig 5. Characterization and reduced immunogenicity through glycosylation of the trimeric component of the I53-50 two-component protein nanoparticle scaffold that is used in clinical-stage subunit protein nanoparticle immunogens.
a. Schematic of protein design models. On the left, twenty I53-50A trimers (gray) and twelve I53-50B pentamers (orange) self-assemble into I53-50 protein nanoparticles [51]. Rosetta sugarcoating design protocols were used to glycosylate the outer surface of I53-50A trimers with 4 N-linked glycans (green) per protomer to form I53-50 particles with 240 N-linked glycans (middle). The inset on the right is a close-up view of glycosylated I53-50A trimers with 12 total glycans on the outward-facing surface. b. Characterization of bare versus glycosylated I53-50 particles using negative stain transmission electron microscopy (nsTEM; scale bar, 100 nm), SDS-PAGE, dynamic light scattering (DLS), and size exclusion chromatography (SEC) on a Superose 6 Increase 10/300 GL column (GE Healthcare). In the SEC chromatogram, both I53-50 and I53-50(gly) particles reach peak elution at 12.5 mL; unassembled I53-50A and I53-50B components elute at ~18 mL. c. ELISA curves (left two plots) and corresponding EC50 titers (right bar plot) showing reduction in anti-I53-50A antibody responses when mice were immunized with I53-50(gly) versus I53-50. BALB/c mice were immunized intramuscularly at 0, 3, and 6 weeks with 5.57 μg of I53-50 or I53-50(gly) and serum antibody binding to I53-50A trimer (left) or I53-50A(gly) trimer (right) was quantified via ELISA using 8-week sera (N = 5 mice/group). For statistical analysis, Mann-Whitney tests were used to compare among the experimental groups.
When glycosylated I53-50A trimers and I53-50B pentamers were mixed in vitro at equimolar concentrations, the two components self-assembled into I53-50(gly) nanoparticles that display 240 glycans on the outer surface (Fig 5A and 5B). Biophysical characterization by negative stain transmission microscopy (nsTEM), dynamic light scattering (DLS), and size exclusion chromatography (SEC) confirmed the formation of monodisperse particles with the known I53-50 morphology (Fig 5B). SDS-PAGE analysis of the I53-50A(gly) trimer treated with PNGase F confirmed that the designed glycans were present in the protein (Fig 5B). Further in vitro characterization and antibody responses against these glycosylated I53-50A trimers has been recently described in other reports [13]. Mice were immunized three times with 5.57 μg of I53-50 or I53-50(gly) particles. Anti-I53-50A trimer serum antibody titers were significantly lower in mice immunized with I53-50(gly) particles compared to mice immunized with I53-50 particles, whereas anti-I53-50A(gly) trimer titers were unchanged between the two groups (Figs 5C and S5). These data demonstrate that the methods presented here can be used for glycan masking through design and analysis of potential sequon motifs and the spatial arrangement of putative glycans on protein surfaces.
Discussion
The GlycanTreeModeler and associated tools allow modelers to accurately model glycans of interest through de novo and density-guided modeling. The algorithm and energy function were rigorously optimized and benchmarked with glycans of varying length and complexity at a median de novo RMSD of 2.7A. In fact, even before full optimization and release, the GlycanSampler algorithm (previously the glycan_relax app) was used to model glycans on HIV [52], Hepatitis C [53], vaccine candidates [54] [55], and (with the final optimized version) SARS-CoV-2 [56], illustrating the general utility of the algorithm and its potential to inform chemical biology.
The modular nature of Rosetta and the tools created for this work allow them to be used in a variety of complex modeling and design tasks. The GlycanTreeModeler was used with previously published density tools [42] to build glycans into their crystallographic or cryoEM experimental density with sub-Angstrom accuracy. However, while the results are encouraging, a truly automated solution for glycoprotein modeling must also sample glycan chemistries, branching, and kinematics simultaneously in order to build potential glycan residues into the density of unknown glycans. Knowledge of the range of glycoforms and occupancy occurring at a glycosylation site can be obtained through mass-spectroscopy techniques [21] [57], but due to chemical and structural heterogeneity at any single glycan site, modelers will typically need to build models for multiple different glycoforms at a single site, especially for complex glycans. The tools presented here can sample and build multiple potential whole glycans at a site through the SimpleGlycosylateMover, but core Rosetta methods that also consider species and cell-type dependent glycan chemistries during the GlycanTreeModeler or end-to-end deep learning methods would be a welcome addition to the methods presented here.
By combining the tools through RosettaScripts, it becomes possible to computationally design glycan sequons at ideal positions on a protein, and then build and model multiple potential glycans at a variety of sites in a symmetric manner. This general workflow was used to sugarcoat a clinically relevant nanoparticle vaccine scaffold with N-linked glycans. In vitro and in vivo testing of this glycosylated scaffold showed a decrease in the humoral immune response to the glycan-masked surface. Sugar coating therapeutics using these methods could potentially reduce off-target effects of many preclinical biologics, especially with respect to immunogenicity.
Most glycans can sample a wide range of conformations in solution, as they are mostly polar, usually exposed to solvent, and have many conformational degrees of freedom. Thus, accurately predicting the lowest energy states (and highest occupancy conformations) for glycans is difficult. In addition, these glycans may be forced into higher-energy internal states through local and crystal contacts. While we can generalize that low energy conformations found through the GlycanTreeModeler should be indicative of probable solution conformations, the GlycanTreeModeler was not benchmarked on an experimental ensemble of glycan structures. The few glycan ensembles found through solution NMR [58] may approximate conformational ensembles in solution and could be the bases for future benchmarking and protocol/scorefunction optimization. However, even with this consideration, many of the benchmark glycans that were modeled accurately to their crystal structures are not hindered by monomer or crystal contacts, but have few interactions to protein residues in their glycan root. Additionally, predictions of the internal (superimposed) RMSDs of all glycans benchmarked were generally favorable with a median benchmarked accuracy of 1.1 Å and a mean of 2.7 Å, indicating that the glycan root, subsequent torsional preferences, and intra-glycan interactions may be determining structural factors for these isolated glycans.
Although the algorithm is capable of accurate de novo modeling of many glycans (especially at their base) and has been used for experimental glycan masking, there is certainly room for improvement. In nearly all of the benchmarks, the native structure is sampled adequately, but in a subset of structures, the energy function is not able to choose near-native structures. Upon further investigation of the many native glycans in the benchmark set with water-mediated hydrogen bonds, we originally hypothesized that explicit water modeling might help the energy function discriminate near-native models. However, we found that implicit modeling actually led to better discrimination scores through the pNear metric. In order to improve the algorithm further, the Rosetta energy function will need to be optimized to improve glycan-protein interactions, specifically in terms of hydrogen bonds, solvation, and the introduction of energy terms that better represent aromatic CH-π interactions [44]. Finally, the algorithm requires more compute time as the number of glycans to model increases, which can be prohibitive for large, multimeric glycoproteins such as HIV.
In this work, optimization of both sampling and scoring was necessary to improve overall accuracy. A key component of the algorithm is the nature-inspired kinematics used during sampling, which was shown to be an important determinant of the overall accuracy of the algorithm. The kinematics were rigorously benchmarked here, though kinematics are not always taken into account or optimized in state-of-the-art classical modeling algorithms. This benchmarking was made possible by the SimpleMetric framework and a new RosettaScripts application that were created and used continuously throughout this work. In addition, we demonstrated the usability of these methods through glycan masking the trimeric subunit of a two-component self-assembling protein nanoparticle that is used as a scaffold to multi-valency display viral glycoprotein antigens. While the glycan masking did not completely remove antibodies specific for the trimer, the experimental results did show proof-of-concept that glycan masking can significantly reduce antibody responses.
SimpleMetrics have now become a critical tool for general analysis in Rosetta and as a way to export important information for external algorithms, such as the quantum annealer [59]. As core protocols in Rosetta continue to be optimized, and as deep learning becomes a more integral aspect of modeling and design, SimpleMetrics should allow the robust analysis of new protocols, results, and Rosetta benchmarks, as it has for this work.
These results show that the GlycanTreeModeler is able to accurately predict glycan structures de novo, build them into known density, and be used in SugarCoating protein surfaces. In addition, the modular nature of the components allows them to be further developed for specific engineering tasks such as immunogenicity reduction or the optimization of developability characteristics such as half-life, solubility, and aggregation potential.
Methods
The Rosetta GlycanTreeModeler builds whole glycan “trees” through an algorithm that mimics the growth of natural trees. A primary difficulty in de novo glycan modeling is the correct prediction of the base of glycoconjugate structures. To increase the accuracy of the first few sugars of the tree, our algorithm begins modeling from the “root” (reducing end) of the glycan tree out to the branching “foliage”. Monte Carlo optimization through sampling of glycan degrees of freedom (DOFs) is carried out through the new GlycanSampler, which includes routines for glycosidic torsion angle (backbone) sampling, structure minimization, hydroxyl and other side-chain optimization, and neighbor protein side-chain optimization. During the protocol, the total amount of sampling scales linearly with the number of glycan residues being modeled, ensuring even sampling regardless of the size or quantity of glycans being modeled.
The GlycanSampler optimizes glycosidic torsion angles using statistically favorable sets of phi, psi, and omega angles (conformers) and single torsions sampled from QM-derived probabilities originally used for energetic evaluation of glycosidic linkages [27] [31],. Conformer sets are dependent on each chemically distinct pair of saccharides making up a glycosidic bond, whereas single torsions depend on the anomeric chemistry of the linkage. We derived the conformers for this work by carrying out a new bioinformatic analysis of glycans in the PDB through the use of adaptive kernel density estimates in a similar manner to what was done for the 2010 Dunbrack Backbone-dependent Rotamer Library [60] (S1 Text).
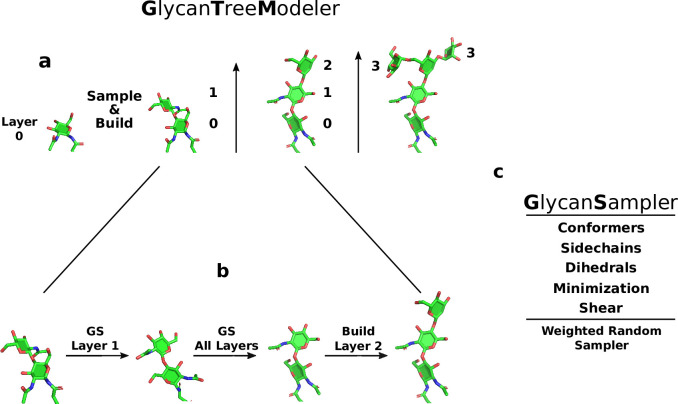
To optimize the conformations of glycan residues on different branches at the same time, the glycan tree is built layer-by-layer, with a layer defined as the residue distance to the root (Fig 6A). Once each new layer is built and optimized, all previous layers are then optimized further (Fig 6B). After all layers are built and optimized, a final optimization is conducted. The lowest energy model (decoy) found during this Monte Carlo algorithm is output at the end of the program as a PDB file. The lowest-energy structure of all the output decoys is used as the “best” model produced by the algorithm (S1 Text).
Fig 6. Glycan modeling diagram.
a. Glycan trees building layer by layer. Numbers indicate distance to root of the glycan tree, which is the first residue. b. After a layer is built, Glycan Sampling is performed on the new layer, and then all layers, before building the next layer. c. Diagram showing major components of the GlycanSampler. The GS is a weighted random sampler, indicating that each DOF is sampled with a specific probability (S1 Text).
Benchmarking protocol
Benchmarking was carried out through the SimpleMetrics framework developed for this work. A SimpleMetric takes a structure and returns a metric or set of metrics, which can then be written to an output scorefile at the end of the protocol during a RosettaScripts execution. A number of SimpleMetric types were developed for textual, numeric, coupled, and per-residue data (S7 Table). These metrics enable calculation of RMSDs, Solvent-Accessible-Surface-Area (SASA), complex hydrogen bonding networks, and other biophyisical properties. These metrics can also be used on-the-fly with Rosetta filters using the SimpleMetricFilter and simple calculations of per-residue data can be achieved using the ResidueSummaryMetric. Many of these metrics were used for benchmarking and analysis (S1 Text). Further, a new application, rosetta_scripts_jd3 was created to enable large-scale benchmarking of Rosetta protocols. This application enables parallel-execution of different rosettascript protocols in parallel, with all resulting experiments tagged during score-file output. This allows for an entire experimental benchmarking pipeline to be created, run, and analyzed through a single Rosetta execution. The Python scripting language was used to load the resulting JSON scorefile for data analysis and figure creation using the numpy [61], pandas [62], and seaborn [63] libraries. All protocol components and their availability in RosettaScripts is listed in S8 Table.
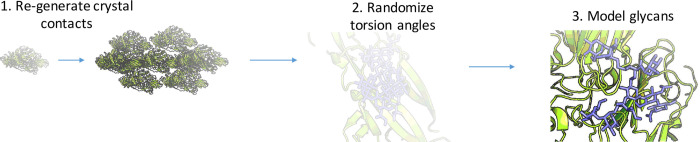
To assess the predictive capability of the GlycanTreeRelax algorithm, the dihedral angles of the glycans are randomized at the start of the algorithm, and waters are removed. Models are compared to the crystal structures using the all-heavy-atom Root Mean Square Deviation (RMSD) metric, with the lowest energy model of all output decoys used for assessment (Fig 7). The RMSD is calculated on all glycan residues that have an acceptable fit to the density in the native model, as terminal glycan residues of some glycans often cannot be observed in the density due to their higher flexibility. A description of the methods used for the RMSD calculation is provided in S1 Text.
Fig 7. Schematic of benchmarking protocol.
Glycan masking
Glycan masking was carried out through the use of two new RosettaScript components; the CreateGlycanSequonMover, which designs typical and enhanced [64] [17], glycan sequons into a protein at a desired position, and the SimpleGlycosylateMover, which adds whole glycans of a given IUPAC onto a protein. Glycans were then sampled using the GlycanTreeModeler through RosettaScripts at each potential glycan position individually. Low-energy and non-clashing models were used to select optimal positions for experimental validation with sequon sequences designed for each position using the CreateGlycanSequonMover (S1 Text).
Availability and Documentation
The GlycanTreeModeler, GlycanSampler, and all tools used in this work are available in the Rosetta Software Suite, which is free for non-commercial use. All tools are available as components for RosettaScripts and PyRosetta. In addition, the use of all core components are covered in publicly accessible tutorials [65] and detailed protocol captures [66]. Results of this study are continuously benchmarked using the Rosetta automated scientific testing framework [67].
Figures
Figures were created using matplotlib [68]. Glycans were visualized in PyMol using the Azahar plugin [69], which was expanded for this work. The cartoonize command was generally run for figures (cartoonize A) for chain A: https://github.com/BIOS-IMASL/Azahar/pull/17
Documentation Links:
- Chapter 13 of the PyRosetta Notebooks:
Supporting information
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(TIF)
Shown is the top 10% of models by total energy. Blue line is the scored native structure with symmetry.
(TIF)
Shown is the top 10% of models by total energy. Blue line is the scored native structure with symmetry.
(TIF)
F241, 243F, 262V, and 264V are shown as spheres at the glycan interface.
(TIF)
(a,b) anti-I53-50A trimer or (c,d) anti-I53-50(gly) trimer antibody responses from mice immunized with (a,c) I53-50 nanoparticles (NP) or (b,d) I53-50(gly) NP.
(TIF)
(TIF)
The von Mises kernel allows for continuous circular description of the torsion angle distribution. The experimental angles from a sample are shown with small dots at the bottom. Such 1-D density estimates were performed for each torsion comprising a glycan-glycan or amino-acid-glycan linkage type. These 64 linkage types can be found in the resulting conformer table included in S1 Data.
(TIF)
All experiments were conducted with the same total amount of sampling. a. Boxplots at decoy enrichments of <1A, <2.5A, and <5.0A. First figure has mean only since most are grouped at zero. b. decoy enrichments of <1A, <2.5A, and <5.0A. Asterisks indicate statistically significant differences through paired t-test. Asterisk above bar indicate statistical significance with all other groups. *,p < .05; **,p < .005; ***,p < .0005.
(TIF)
(TIF)
(TIF)
N = 150,000; 37,500 per experiment.
(TIF)
a. Kernel Density Estimates of enrichment per input model for each major kinematic experiment. b. Box plot of enrichment of each input model per experiment less than 7.5 A RMSD to the native crystal structure. C. Means of B, with paired t-test, All vs. All. * indicates p < .05. p-value for hybrid-build-one vs. build-by-layer p< .005, while vs. all-sampler p < .0005.
(TIF)
a. Boxplots of each input glycan benchmark at <1.0A, <2.5A, and < 5.0A of the glycan STEM b. Means of panel a. Asterisk above bar indicate statistical significance with all other groups through paired t-test. *|p < .05; **|p < .005; ***|p < .0005. For b <1A, pvalue of all-sampler vs. hybrid-build-one is **.
(TIF)
1000 decoys were produced for each glycan and each experiment for a total of 125k decoys. Note that this is a third less than all other optimization experiments. Boxplots and barplots of PNear metric at each significant lambda are shown. Blue squares indicate mean. Line in box indicates median. Upper left figure shows only means as most datapoints are grouped at 0 and the box could not be seen. Paired T-test results between sugar_bb weight of 1.0 and .5 are indicated.
(TIF)
a. Boxplot of pNear values for each benchmark glycan, indicating funnel plot quality for lambdas of 1.0, 2.5, and 5.0 RMSD to native. Higher pNear indicates better near-native discrimination from other decoys. Blue squares indicate mean. b. Means of pNear over each experiment. Significance from paired t-test; * indicates p < .05.
(TIF)
Asterisk above bar indicate statistical significance with all other groups through paired t-test. *|p < .05 **|p < .005 ***|p < .0005 a. Decoy Enrichment in output models at <1.0A, <2.5A, and <5.0A RMSD. b. Decoy Enrichment in output models of the base (STEM) region indicating layers 0 and 1.
(TIF)
(TIF)
Please see README for a full description of each component. Briefly, these include the following scripts: create_substituted_JD.py—Used to create substituted job description file for rosetta_scripts_jd3; create_symmetry_mates.py—This was used to create symmetry mates with a 12 A radius; density_build_example.xml—An an example of a main job definition file before being substituted for each glycan; density_build_example_substituted.xml—Substituted version; glycan_bm_plots.py—Tools used for plotting all benchmark experiments from Rosetta JSON files.; glycan_conformer_table.txt—The resultant glycan conformer table used in Rosetta and benchmarked against; glycan_tree_relax.xml—Main script used for de novo benchmarking.; glycan_tree_relax_dens.xml—Main script used for density building.; glycan_tree_relax_with_solvation.xml—Script used for running the GTM with explicit waters; glycan_water_mediated_hbonds.xml—Script for calculating water mediated hydrogen bonds; pdb_roots_density_sym.txt—Benchmark glycan list used for create_substituted_JD.py; refine_with_map_dih2.xml—Script used for refining input structures into the rosetta energy function using density.
(ZIP)
(MOV)
Acknowledgments
We gratefully acknowledge Rashmi Ravichandran for providing I53-50B pentamer, Deleah Pettie and Michael Murphy for assistance with expression of I53-50A(gly) design models, Alex Roederer for preparation of I53-50 and I53-50(gly) particles for mouse immunization, and Minh N Pham for performing mouse immunizations and blood draws.
Data Availability
All main methods developed in this manuscript are part of the Rosetta software suite, which is free for academic use with full code released (https://www.rosettacommons.org). All associated scripts are available as supplemental or attached as text in the supplemental section.
Funding Statement
This work was supported by NIAID grants U19AI117905, R01AI11386, 1UM1 AI100663, and UM1 AI144462 to WRS, by PATH Malaria Vaccine Initiative under Grant OPP1141162 from the Bill & Melinda Gates Foundation to WRS, by BMGF CAVD funding to the IAVI NAC Center to WRS, by Grant OPP1156262 from the Bill & Melinda Gates Foundation and a generous donation from the Open Philanthropy Project to NPK, NIH awards 1P01AI167966 and P50AI150464 to NPK, and by an NIAID training grant fellowship T32AI007244 to JAB. NIH award R01-GM127278 supported JWL and JJG. NIH/NIGMS R35 GM122517 (R. Dunbrack) supported MS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ernst B, Hart GW, Sinaý P. Carbohydrates in Chemistry and Biology [Internet]. 1st ed. Wiley; 2000. [cited 2020 Dec 18]. Available from: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527618255. [Google Scholar]
- 2.Ploegh HL. Viral Strategies of Immune Evasion. Science. 1998. Apr 10;280(5361):248–53. doi: 10.1126/science.280.5361.248 [DOI] [PubMed] [Google Scholar]
- 3.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, et al. A Potent and Broad Neutralizing Antibody Recognizes and Penetrates the HIV Glycan Shield. Science. 2011. Nov 25;334(6059):1097–103. doi: 10.1126/science.1213256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julien JP, Sok D, Khayat R, Lee JH, Doores KJ, Walker LM, et al. Broadly Neutralizing Antibody PGT121 Allosterically Modulates CD4 Binding via Recognition of the HIV-1 gp120 V3 Base and Multiple Surrounding Glycans. Trkola A, editor. PLoS Pathog. 2013. May 2;9(5):e1003342. doi: 10.1371/journal.ppat.1003342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falkowska E, Le KM, Ramos A, Doores KJ, Lee JH, Blattner C, et al. Broadly Neutralizing HIV Antibodies Define a Glycan-Dependent Epitope on the Prefusion Conformation of gp41 on Cleaved Envelope Trimers. Immunity. 2014. May;40(5):657–68. doi: 10.1016/j.immuni.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells L. Glycosylation of Nucleocytoplasmic Proteins: Signal Transduction and O-GlcNAc. Science. 2001. Mar 23;291(5512):2376–8. doi: 10.1126/science.1058714 [DOI] [PubMed] [Google Scholar]
- 7.Jennewein MF, Alter G. The Immunoregulatory Roles of Antibody Glycosylation. Trends in Immunology. 2017. May;38(5):358–72. doi: 10.1016/j.it.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 8.Irvine EB, Alter G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology. 2020. Mar 20;30(4):241–53. doi: 10.1093/glycob/cwaa018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shental-Bechor D, Levy Y. Effect of glycosylation on protein folding: A close look at thermodynamic stabilization. Proceedings of the National Academy of Sciences. 2008. Jun 17;105(24):8256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinclair AM, Elliott S. Glycoengineering: The effect of glycosylation on the properties of therapeutic proteins. Journal of Pharmaceutical Sciences. 2005. Aug;94(8):1626–35. doi: 10.1002/jps.20319 [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Nema S, Teagarden D. Protein aggregation—Pathways and influencing factors. International Journal of Pharmaceutics. 2010. May;390(2):89–99. doi: 10.1016/j.ijpharm.2010.02.025 [DOI] [PubMed] [Google Scholar]
- 12.Duan H, Chen X, Boyington JC, Cheng C, Zhang Y, Jafari AJ, et al. Glycan Masking Focuses Immune Responses to the HIV-1 CD4-Binding Site and Enhances Elicitation of VRC01- Class Precursor Antibodies. Immunity. 2018. Aug;49(2):301–311.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraft JC, Pham MN, Shehata L, Brinkkemper M, Boyoglu-Barnum S, Sprouse KR, et al. Antigen- and scaffold-specific antibody responses to protein nanoparticle immunogens. Cell Rep Med. 2022. Oct 18;3(10):100780. doi: 10.1016/j.xcrm.2022.100780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Read BJ, Won L, Kraft JC, Sappington I, Aung A, Wu S, et al. Mannose-binding lectin and complement mediate follicular localization and enhanced immunogenicity of diverse protein nanoparticle immunogens. Cell Rep. 2022. Jan 11;38(2):110217. doi: 10.1016/j.celrep.2021.110217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rini JM, Esko JD. Glycosyltransferases and Glycan-Processing Enzymes. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of Glycobiology [Internet]. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015. [cited 2020 Dec 18]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK453021/. [Google Scholar]
- 16.Rao RSP, Wollenweber B. Do N-glycoproteins have preference for specific sequons? Bioinformation. 2010. Nov 1;5(5):208–12. doi: 10.6026/97320630005208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YW, Yang HI, Wu YT, Hsu TL, Lin TW, Kelly JW, et al. Residues Comprising the Enhanced Aromatic Sequon Influence Protein N-Glycosylation Efficiency. J Am Chem Soc. 2017. Sep 20;139(37):12947–55. doi: 10.1021/jacs.7b03868 [DOI] [PubMed] [Google Scholar]
- 18.Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers. Biochimica et Biophysica Acta (BBA)—General Subjects. 2012. Sep;1820(9):1347–53. doi: 10.1016/j.bbagen.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 19.Haltiwanger RS, Lowe JB. Role of Glycosylation in Development. Annu Rev Biochem. 2004. Jun;73(1):491–537. doi: 10.1146/annurev.biochem.73.011303.074043 [DOI] [PubMed] [Google Scholar]
- 20.Suga A, Nagae M, Yamaguchi Y. Analysis of protein landscapes around N-glycosylation sites from the PDB repository for understanding the structural basis of N-glycoprotein processing and maturation. Glycobiology. 2018. Oct 1;28(10):774–85. doi: 10.1093/glycob/cwy059 [DOI] [PubMed] [Google Scholar]
- 21.Riley NM, Hebert AS, Westphall MS, Coon JJ. Capturing site-specific heterogeneity with large-scale N-glycoproteome analysis. Nat Commun. 2019. Dec;10(1):1311. doi: 10.1038/s41467-019-09222-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren WW, Jin ZC, Dong W, Kitajima T, Gao XD, Fujita M. Glycoengineering of HEK293 cells to produce high-mannose-type N-glycan structures. The Journal of Biochemistry. 2019. Sep 1;166(3):245–58. doi: 10.1093/jb/mvz032 [DOI] [PubMed] [Google Scholar]
- 23.Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging Principles for the Therapeutic Exploitation of Glycosylation. Science. 2014. Jan 3;343(6166):1235681. doi: 10.1126/science.1235681 [DOI] [PubMed] [Google Scholar]
- 24.Woods RJ. Predicting the Structures of Glycans, Glycoproteins, and Their Complexes. Chem Rev. 2018. Sep 12;118(17):8005–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Ozorowski G, Ward AB. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science. 2016. Mar 4;351(6277):1043–8. doi: 10.1126/science.aad2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pallesen J, Murin CD, de Val N, Cottrell CA, Hastie KM, Turner HL, et al. Structures of Ebola virus GP and sGP in complex with therapeutic antibodies. Nat Microbiol. 2016. Sep;1(9):16128. doi: 10.1038/nmicrobiol.2016.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nivedha AK, Makeneni S, Foley BL, Tessier MB, Woods RJ. Importance of ligand conformational energies in carbohydrate docking: Sorting the wheat from the chaff. J Comput Chem. 2014. Mar 15;35(7):526–39. doi: 10.1002/jcc.23517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohne-Lang A, von der Lieth CW. GlyProt: in silico glycosylation of proteins. Nucleic Acids Research. 2005. Jul 1;33(Web Server):W214–9. doi: 10.1093/nar/gki385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lütteke T. pdb-care (PDB CArbohydrate REsidue check): a program to support annotation of complex carbohydrate structures in PDB files. BMC Bioinformatics. 2004;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank M, Lutteke T, von der Lieth CW. GlycoMapsDB: a database of the accessible conformational space of glycosidic linkages. Nucleic Acids Research. 2007. Jan 3;35(Database):287–90. doi: 10.1093/nar/gkl907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nivedha AK, Thieker DF, Makeneni S, Hu H, Woods RJ. Vina-Carb: Improving Glycosidic Angles during Carbohydrate Docking. J Chem Theory Comput. 2016. Feb 9;12(2):892–901. doi: 10.1021/acs.jctc.5b00834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nance ML, Labonte JW, Adolf-Bryfogle J, Gray JJ. Development and Evaluation of GlycanDock: A Protein–Glycoligand Docking Refinement Algorithm in Rosetta. J Phys Chem B. 2021. Jun 16;acs.jpcb.1c00910. doi: 10.1021/acs.jpcb.1c00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirschner KN, Yongye AB, Tschampel SM, González-Outeiriño J, Daniels CR, Foley BL, et al. GLYCAM06: A generalizable biomolecular force field. Carbohydrates: GLYCAM06. J Comput Chem. 2008. Mar;29(4):622–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emsley P, Crispin M. Structural analysis of glycoproteins: building N-linked glycans with Coot. Acta Crystallogr D Struct Biol. 2018. Apr 1;74(4):256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park SJ, Lee J, Qi Y, Kern NR, Lee HS, Jo S, et al. CHARMM-GUI Glycan Modeler for modeling and simulation of carbohydrates and glycoconjugates. Glycobiology. 2019. Apr 1;29(4):320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leman JK, Weitzner BD, Lewis SM, Adolf-Bryfogle J, Alam N, Alford RF, et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks. Nat Methods. 2020. Jul;17(7):665–80. doi: 10.1038/s41592-020-0848-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleishman SJ, Leaver-Fay A, Corn JE, Strauch EM, Khare SD, Koga N, et al. RosettaScripts: A Scripting Language Interface to the Rosetta Macromolecular Modeling Suite. Uversky VN, editor. PLoS ONE. 2011. Jun 24;6(6):e20161. doi: 10.1371/journal.pone.0020161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray JJ, Chaudhury S, Lyskov S. The PyRosetta Interactive Platform for Protein Structure Prediction and Design. 2009. [cited 2014 Mar 26]; Available from: http://graylab.jhu.edu/~sid/pyrosetta/downloads/documentation/PyRosetta_Textbook.pdf. [Google Scholar]
- 39.Alford RF, Leaver-Fay A, Jeliazkov JR, O’Meara MJ, DiMaio FP, Park H, et al. The Rosetta All-Atom Energy Function for Macromolecular Modeling and Design. J Chem Theory Comput. 2017. Jun 13;13(6):3031–48. doi: 10.1021/acs.jctc.7b00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labonte JW, Adolf-Bryfogle J, Schief WR, Gray JJ. Residue-centric modeling and design of saccharide and glycoconjugate structures. J Comput Chem. 2017. Feb 15;38(5):276–87. doi: 10.1002/jcc.24679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frenz B, Rämisch S, Borst AJ, Walls AC, Adolf-Bryfogle J, Schief WR, et al. Automatically Fixing Errors in Glycoprotein Structures with Rosetta. Structure. 2019. Jan;27(1):134–139.e3. doi: 10.1016/j.str.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiMaio F, Tyka MD, Baker ML, Chiu W, Baker D. Refinement of Protein Structures into Low-Resolution Density Maps Using Rosetta. Journal of Molecular Biology. 2009. Sep;392(1):181–90. doi: 10.1016/j.jmb.2009.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhardwaj G, Mulligan VK, Bahl CD, Gilmore JM, Harvey PJ, Cheneval O, et al. Accurate de novo design of hyperstable constrained peptides. Nature. 2016. Oct;538(7625):329–35. doi: 10.1038/nature19791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hudson KL, Bartlett GJ, Diehl RC, Agirre J, Gallagher T, Kiessling LL, et al. Carbohydrate–Aromatic Interactions in Proteins. J Am Chem Soc. 2015. Dec 9;137(48):15152–60. doi: 10.1021/jacs.5b08424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlovicz RE, Park H, DiMaio F. Efficient consideration of coordinated water molecules improves computational protein-protein and protein-ligand docking discrimination. Wallner B, editor. PLoS Comput Biol. 2020. Sep 21;16(9):e1008103. doi: 10.1371/journal.pcbi.1008103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed FK, Clark BE, Burton DR, Pantophlet R. An engineered mutant of HIV-1 gp120 formulated with adjuvant Quil A promotes elicitation of antibody responses overlapping the CD4-binding site. Vaccine. 2012. Jan;30(5):922–30. doi: 10.1016/j.vaccine.2011.11.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin SC, Liu WC, Jan JT, Wu SC. Glycan Masking of Hemagglutinin for Adenovirus Vector and Recombinant Protein Immunizations Elicits Broadly Neutralizing Antibodies against H5N1 Avian Influenza Viruses. Kang SM, editor. PLoS ONE. 2014. Mar 26;9(3):e92822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garrity RR, Rimmelzwaan G, Minassian A, Tsai WP, Lin G, de Jong JJ, et al. Refocusing neutralizing antibody response by targeted dampening of an immunodominant epitope. J Immunol. 1997. Jul 1;159(1):279–89. [PubMed] [Google Scholar]
- 49.Walls AC, Fiala B, Schäfer A, Wrenn S, Pham MN, Murphy M, et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell. 2020. Nov 25;183(5):1367–1382.e17. doi: 10.1016/j.cell.2020.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcandalli J, Fiala B, Ols S, Perotti M, de van der Schueren W, Snijder J, et al. Induction of Potent Neutralizing Antibody Responses by a Designed Protein Nanoparticle Vaccine for Respiratory Syncytial Virus. Cell. 2019. Mar 7;176(6):1420–1431.e17. doi: 10.1016/j.cell.2019.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bale JB, Gonen S, Liu Y, Sheffler W, Ellis D, Thomas C, et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science. 2016. Jul 22;353(6297):389–94. doi: 10.1126/science.aaf8818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ringe RP, Cruz Portillo VM, Dosenovic P, Ketas TJ, Ozorowski G, Nogal B, et al. Neutralizing Antibody Induction by HIV-1 Envelope Glycoprotein SOSIP Trimers on Iron Oxide Nanoparticles May Be Impaired by Mannose Binding Lectin. Silvestri G, editor. J Virol. 2019. Dec 18;94(6):e01883–19, /jvi/94/6/JVI.01883-19.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urbanowicz RA, Wang R, Schiel JE, Keck Z yong, Kerzic MC, Lau P, et al. Antigenicity and Immunogenicity of Differentially Glycosylated Hepatitis C Virus E2 Envelope Proteins Expressed in Mammalian and Insect Cells. James Ou JH, editor. J Virol. 2019. Jan 16;93(7):e01403–18, /jvi/93/7/JVI.01403-18.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Havenar-Daughton C, Sarkar A, Kulp DW, Toy L, Hu X, Deresa I, et al. The human naive B cell repertoire contains distinct subclasses for a germline-targeting HIV-1 vaccine immunogen. Sci Transl Med. 2018. Jul 4;10(448):eaat0381. doi: 10.1126/scitranslmed.aat0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Z, Wise MC, Chokkalingam N, Walker S, Tello-Ruiz E, Elliott STC, et al. In Vivo Assembly of Nanoparticles Achieved through Synergy of Structure-Based Protein Engineering and Synthetic DNA Generates Enhanced Adaptive Immunity. Adv Sci. 2020. Apr;7(8):1902802. doi: 10.1002/advs.201902802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gowthaman R, Guest JD, Yin R, Adolf-Bryfogle J, Schief WR, Pierce BG. CoV3D: a database of high resolution coronavirus protein structures. Nucleic Acids Research. 2021. Jan 8;49(D1):D282–7. doi: 10.1093/nar/gkaa731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao L, Diedrich JK, Ma Y, Wang N, Pauthner M, Park SKR, et al. Global site-specific analysis of glycoprotein N-glycan processing. Nat Protoc. 2018. Jun;13(6):1196–212. doi: 10.1038/nprot.2018.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freedberg DI, Kwon J. Solution NMR Structural Studies of Glycans. Isr J Chem. 2019. Nov;59(11–12):1039–58. [Google Scholar]
- 59.Mulligan VK, Melo H, Merritt HI, Slocum S, Weitzner BD, Watkins AM, et al. Designing Peptides on a Quantum Computer [Internet]. Bioengineering; 2019. Sep [cited 2021 Jan 12]. Available from: http://biorxiv.org/lookup/doi/10.1101/752485. [Google Scholar]
- 60.Shapovalov MV, Dunbrack RL. A Smoothed Backbone-Dependent Rotamer Library for Proteins Derived from Adaptive Kernel Density Estimates and Regressions. Structure. 2011. Jun;19(6):844–58. doi: 10.1016/j.str.2011.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, et al. Array programming with NumPy. Nature. 2020. Sep 17;585(7825):357–62. doi: 10.1038/s41586-020-2649-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKinney W. Data Structures for Statistical Computing in Python. In Austin, Texas; 2010. [cited 2021 Jan 7]. p. 56–61. Available from: https://conference.scipy.org/proceedings/scipy2010/mckinney.html. [Google Scholar]
- 63.Waskom M, Gelbart M, Botvinnik O, Ostblom J, Hobson P, Lukauskas S, et al. mwaskom/seaborn: v0.11.1 (December 2020) [Internet]. Zenodo; 2020. [cited 2021 Jan 7]. Available from: https://zenodo.org/record/592845. [Google Scholar]
- 64.Murray AN, Chen W, Antonopoulos A, Hanson SR, Wiseman RL, Dell A, et al. Enhanced Aromatic Sequons Increase Oligosaccharyltransferase Glycosylation Efficiency and Glycan Homogeneity. Chemistry & Biology. 2015. Aug;22(8):1052–62. doi: 10.1016/j.chembiol.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le K, Adolf-Bryfogle J, Klima J, Lyskov S, Labonte J, Bertolani S, et al. PyRosetta Jupyter Notebooks Teach Biomolecular Structure Prediction and Design [Internet]. ENGINEERING; 2020. Feb [cited 2021 Jan 7]. Available from: https://www.preprints.org/manuscript/202002.0097/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schoeder CT, Schmitz S, Adolf-Bryfogle J, Sevy AM, Finn JA, Sauer MF, et al. Modeling Immunity with Rosetta: Methods for Antibody and Antigen Design. Biochemistry. 2021. Mar 11;acs.biochem.0c00912. doi: 10.1021/acs.biochem.0c00912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koehler Leman J, Lyskov S, Lewis S, Adolf-Bryfogle J, Alford RF, Barlow K, et al. Ensuring scientific reproducibility in bio-macromolecular modeling via extensive, automated benchmarks [Internet]. Bioinformatics; 2021. Apr [cited 2021 Sep 27]. Available from: http://biorxiv.org/lookup/doi/ doi: 10.1101/2021.04.04.438423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hunter JD. Matplotlib: A 2D Graphics Environment. Comput Sci Eng. 2007;9(3):90–5. [Google Scholar]
- 69.Arroyuelo A, Vila JA, Martin OA. Azahar: a PyMOL plugin for construction, visualization and analysis of glycan molecules. J Comput Aided Mol Des. 2016. Aug;30(8):619–24. doi: 10.1007/s10822-016-9944-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(TIF)
Shown is the top 10% of models by total energy. Blue line is the scored native structure with symmetry.
(TIF)
Shown is the top 10% of models by total energy. Blue line is the scored native structure with symmetry.
(TIF)
F241, 243F, 262V, and 264V are shown as spheres at the glycan interface.
(TIF)
(a,b) anti-I53-50A trimer or (c,d) anti-I53-50(gly) trimer antibody responses from mice immunized with (a,c) I53-50 nanoparticles (NP) or (b,d) I53-50(gly) NP.
(TIF)
(TIF)
The von Mises kernel allows for continuous circular description of the torsion angle distribution. The experimental angles from a sample are shown with small dots at the bottom. Such 1-D density estimates were performed for each torsion comprising a glycan-glycan or amino-acid-glycan linkage type. These 64 linkage types can be found in the resulting conformer table included in S1 Data.
(TIF)
All experiments were conducted with the same total amount of sampling. a. Boxplots at decoy enrichments of <1A, <2.5A, and <5.0A. First figure has mean only since most are grouped at zero. b. decoy enrichments of <1A, <2.5A, and <5.0A. Asterisks indicate statistically significant differences through paired t-test. Asterisk above bar indicate statistical significance with all other groups. *,p < .05; **,p < .005; ***,p < .0005.
(TIF)
(TIF)
(TIF)
N = 150,000; 37,500 per experiment.
(TIF)
a. Kernel Density Estimates of enrichment per input model for each major kinematic experiment. b. Box plot of enrichment of each input model per experiment less than 7.5 A RMSD to the native crystal structure. C. Means of B, with paired t-test, All vs. All. * indicates p < .05. p-value for hybrid-build-one vs. build-by-layer p< .005, while vs. all-sampler p < .0005.
(TIF)
a. Boxplots of each input glycan benchmark at <1.0A, <2.5A, and < 5.0A of the glycan STEM b. Means of panel a. Asterisk above bar indicate statistical significance with all other groups through paired t-test. *|p < .05; **|p < .005; ***|p < .0005. For b <1A, pvalue of all-sampler vs. hybrid-build-one is **.
(TIF)
1000 decoys were produced for each glycan and each experiment for a total of 125k decoys. Note that this is a third less than all other optimization experiments. Boxplots and barplots of PNear metric at each significant lambda are shown. Blue squares indicate mean. Line in box indicates median. Upper left figure shows only means as most datapoints are grouped at 0 and the box could not be seen. Paired T-test results between sugar_bb weight of 1.0 and .5 are indicated.
(TIF)
a. Boxplot of pNear values for each benchmark glycan, indicating funnel plot quality for lambdas of 1.0, 2.5, and 5.0 RMSD to native. Higher pNear indicates better near-native discrimination from other decoys. Blue squares indicate mean. b. Means of pNear over each experiment. Significance from paired t-test; * indicates p < .05.
(TIF)
Asterisk above bar indicate statistical significance with all other groups through paired t-test. *|p < .05 **|p < .005 ***|p < .0005 a. Decoy Enrichment in output models at <1.0A, <2.5A, and <5.0A RMSD. b. Decoy Enrichment in output models of the base (STEM) region indicating layers 0 and 1.
(TIF)
(TIF)
Please see README for a full description of each component. Briefly, these include the following scripts: create_substituted_JD.py—Used to create substituted job description file for rosetta_scripts_jd3; create_symmetry_mates.py—This was used to create symmetry mates with a 12 A radius; density_build_example.xml—An an example of a main job definition file before being substituted for each glycan; density_build_example_substituted.xml—Substituted version; glycan_bm_plots.py—Tools used for plotting all benchmark experiments from Rosetta JSON files.; glycan_conformer_table.txt—The resultant glycan conformer table used in Rosetta and benchmarked against; glycan_tree_relax.xml—Main script used for de novo benchmarking.; glycan_tree_relax_dens.xml—Main script used for density building.; glycan_tree_relax_with_solvation.xml—Script used for running the GTM with explicit waters; glycan_water_mediated_hbonds.xml—Script for calculating water mediated hydrogen bonds; pdb_roots_density_sym.txt—Benchmark glycan list used for create_substituted_JD.py; refine_with_map_dih2.xml—Script used for refining input structures into the rosetta energy function using density.
(ZIP)
(MOV)
Data Availability Statement
All main methods developed in this manuscript are part of the Rosetta software suite, which is free for academic use with full code released (https://www.rosettacommons.org). All associated scripts are available as supplemental or attached as text in the supplemental section.