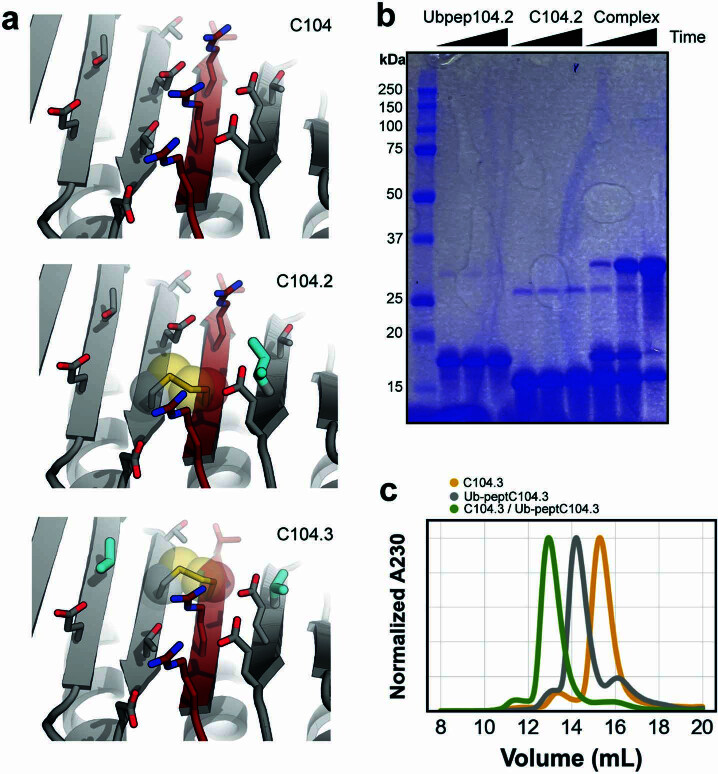
Extended Data Fig. 4. Disulfide functionalization of C104.
a, Close-up of C104 surface exposed interface (top) and of the disulfide bridge variants C104.2 (middle) and C104.3 (bottom). Disulfide bonds are highlighted with spheres while additional redesigned residues to optimally accommodate the disulfide bridges are highlighted in cyan thicker sticks. Designed binder in gray and peptide in dark red. b, Representative coommassie stained non-reducing SDS-PAGE gel monitoring disulfide bridge formation of C104.2. Time points are t=0, t=90min and t=overnight. Experiment was reproduced twice with 2 independent protein preparations c, Superdex 75 increase 10/300 GL SEC binding assay confirming that the cysteine containing peptide of C104.3 fused to ubiquitin can bind to its designed cysteine containing binding partner C104.3.