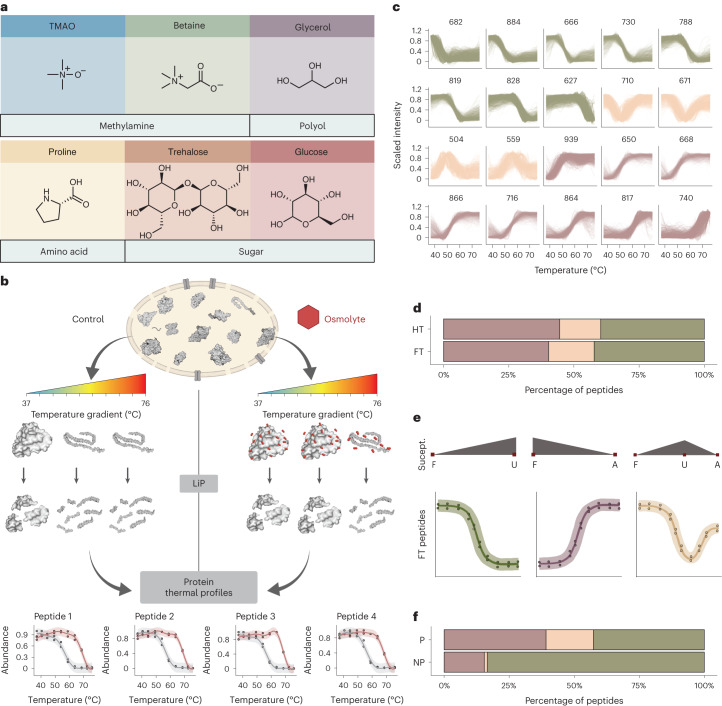
Fig. 1. LiP–MS thermal profiling to study osmolyte effects.
a, Chemical structures of osmolytes used in the study. All osmolytes were used at 1 M except for trehalose (0.5 M). b, Overview of the experimental procedure. Aliquots of an E. coli lysate were subjected to a thermal gradient (ten temperatures; 37–76 °C) in the presence or absence of an osmolyte. Subsequent proteolysis with PK under native conditions yielded proteolytic fragments that are informative about the folded state of proteins, with PK accessibility increasing after protein unfolding. Trypsin digestion under denaturing conditions generated peptides that can be measured with MS. Scaled relative abundance of individual peptides across the temperature gradient allows profiling of protein thermal unfolding, and osmolyte effects can be studied by comparing profiles between control (gray curves) and osmolyte (red curves) conditions. Figure created with BioRender.com. c, Peptide thermal profile clusters in the absence of osmolytes. Both fully tryptic (FT) and half-tryptic (HT) peptides are shown. The numbers of peptides in each cluster are indicated. Colors indicate profiles with decreasing intensity (group 1, green), profiles with increasing intensity (group 2, purple) and nonmonotonous profiles (group 3, orange). d, Percentage of cluster groups from c shown separately for FT and HT peptides. e, Interpretation of FT peptide behavior in the three cluster groups from c. We interpret changing FT peptide intensity as a function of increased or decreased proteolytic susceptibility (Suscept.) as indicating protein in a folded (F), unfolded (U) or aggregated (A) state. For HT peptides, the opposite effect (that is, a flipped thermal profile) is expected (Supplementary Fig. 1f). f, Percentage of cluster groups from c separated by proteins defined as nonprecipitators (NP) and precipitators (P) in TPP; only FT peptides are shown (see Supplementary Fig. 1g for HT peptides).