Abstract
Study Design
Delayed diagnosis of degenerative cervical myelopathy (DCM) is associated with reduced quality of life and greater disability. Developing diagnostic criteria for DCM has been identified as a top research priority.
Objectives
This scoping review aims to address the following questions: What is the diagnostic accuracy and frequency of clinical symptoms in patients with DCM?
Methods
A scoping review was conducted using a database of all primary DCM studies published between 2005 and 2020. Studies were included if they (i) assessed the diagnostic accuracy of a symptom using an appropriate control group or (ii) reported the frequency of a symptom in a cohort of DCM patients.
Results
This review identified three studies that discussed the diagnostic accuracy of various symptoms and included a control group. An additional 58 reported on the frequency of symptoms in a cohort of patients with DCM. The most frequent and sensitive symptoms in DCM include unspecified paresthesias (86%), hand numbness (82%) and hand paresthesias (79%). Neck and/or shoulder pain was present in 51% of patients with DCM, whereas a minority had back (19%) or lower extremity pain (10%). Bladder dysfunction was uncommon (38%) although more frequent than bowel (23%) and sexual impairment (4%). Gait impairment is also commonly seen in patients with DCM (72%).
Conclusion
Patients with DCM present with many different symptoms, most commonly sensorimotor impairment of the upper extremities, pain, bladder dysfunction and gait disturbance. If patients present with a combination of these symptoms, further neuroimaging is indicated to confirm the diagnosis of DCM.
Keywords: degenerative disc disease, cervical, myelopathy
Introduction
Degenerative cervical myelopathy (DCM) is a progressive spine disease and the most common cause of spinal cord impairment worldwide.1,2 In several countries, the pathway to the diagnosis of DCM typically starts at the level of the primary care physician or non-spine specialist. Unfortunately, DCM is underrepresented in medical school or postgraduate curricula, commonly used textbooks and question banks. 3 Furthermore, only 45% of myelopathy symptoms entered into Web-based symptom checkers include DCM as a differential diagnosis. 4 Individuals are therefore unlikely to consider DCM when or before presenting to their primary care physician.
Consequently, diagnosis of DCM is often delayed. A recent study by Hilton et al 5 (2019) investigated the pathway from symptom onset to surgical assessment in the United Kingdom healthcare system. Based on their results, the time between symptom onset and referral by a primary care physician was 8.3 ± 10.1 months for new cases of DCM, representing the greatest delay in the diagnostic pathway. Furthermore, seventy-six percent of new cases were initially referred to a speciality other than spinal surgery such as neurology, pain management, rheumatology and geriatrics. Ultimately, the mean time between symptom onset and surgical evaluation was 17.7 ± 16.0 months. This delay in assessment by a qualified spine provider can have a deleterious effect on neurological and functional recovery following surgery. 6 For instance, based on a study by Pope et al 7 (2020), patients whose diagnosis was made 1-2 years after presentation were more likely to be unable to work and further delays resulted in increased dependence on others for activities of daily living. Additionally, myelopathy severity, duration of symptoms and gait dysfunction are significant predictors of worse surgical outcome, making early detection, as well as identification of milder patients, a priority. 6 Furthermore, DCM results in an estimated annual loss of productivity of £362.6 m, costs £280.2 m in disability benefits and imposes an overall cost to society of £681.6 m. The direct and indirect costs of managing patients with DCM could be reduced with accurate and timely diagnosis. 8 Therefore, it is imperative to shorten the time to diagnosis and improve the pathway of care to definitive management in order to optimize patient outcomes and reduce lifelong disability.
Misdiagnosis or delayed diagnosis of DCM is likely due to the variety of clinical presentations, incomplete neurological examinations by clinicians and reduced awareness of this condition. As DCM results from compression of the cervical spinal cord, patients present with a wide range of sensory and motor complaints in their upper and lower extremities as well as evidence of autonomic dysfunction.9-11 Common complaints include bilateral arm paresthesia, reduced manual dexterity, impaired gait and weakness. 10 Other symptoms (a manifestation of disease apparent to the patient) include neck pain or stiffness, Lhermitte’s phenomena and urgency of urination or defection. On physical examination, patients with DCM exhibit a combination of upper and lower motor neuron signs as well as impaired sensation to light touch, temperature, proprioception, vibration and pain.
Given that there is no single clinical feature or test that is sufficient to diagnose DCM, developing diagnostic criteria for this condition would be invaluable. Diagnostic criteria for DCM could (i) improve patient care by facilitating earlier diagnosis and treatment, (ii) act as reference for primary care physicians, allied health professionals and other specialists who encounters these patients and (iii) serve as a basis for developing a triaging and surveillance system. As part of the AO Spine RECODE-DCM (Research Objectives and Common Data Elements for Degenerative Cervical Myelopathy) project, establishing diagnostic criteria was identified as one of the top ten priorities for future research.12,13 The first step in this process is to determine candidate variables for inclusion in diagnostic criteria. Symptoms that are frequently reported in patients with DCM and exhibit high sensitivity and specificity are important to identify.
The objective of this study is to conduct a scoping review of the literature in order to address the following key questions (KQ):
KQ1: What is the diagnostic accuracy (ie sensitivity, specificity, positive or negative predictive value, positive or negative likelihood ratio) of clinical symptoms in patients with DCM?
KQ2: What is the frequency of clinical symptoms in patients with DCM?
Methods
A scoping review was conducted to assess the diagnostic accuracy of clinical symptoms in patients with DCM. The scoping review was formatted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist. 14 A systematic review was not performed as the evidence on the diagnostic accuracy of symptoms is limited. Neither informed consent nor Institutional Review Board approval were required due to the nature of the study.
Eligibility Criteria
Table 1 summarizes the inclusion and exclusion criteria in terms of population of interest, clinical symptoms, outcomes and study design.
Table 1.
Inclusion and Exclusion Criteria.
| Characteristic | Inclusion | Exclusion |
|---|---|---|
| Population | - Patients with cervical myelopathy secondary to spondylosis, disc herniation, ossification of the posterior longitudinal ligament, congenital stenosis or subluxation. - Managed conservatively or surgically. - Age >18 years |
- Patient with traumatic spinal cord injury, thoracic or lumbar myelopathy, tumor or infection. |
| Symptoms | - Numb hands Clumsy hands - Arm paresthesia - Neck pain - Shoulder or arm pain - Weakness - Gait disturbances - Bladder or bowel dysfunction |
- Clinical signs on physical examination - Patient reported outcome measures (eg neck disability index, SF-36, VAS, subjective questionnaires) - Clinician reported outcome measures (eg mJOA, Nurick, walking test, grip dynamometer, GRASSP, GaitRite) - Imaging characteristics |
| Outcome | KQ1: Sensitivity, specificity, positive or negative predictive, positive or negative likelihood ratio KQ2: Frequency, percentages |
- Reliability - Responsiveness to change - Internal consistency |
| Study design | KQ1: Case-control or cohort studies. Acceptable control group for comparison (eg individuals with cervical radiculopathy or axial neck pain with no myelopathic symptoms) KQ2: Clinical trial or cohort studies that reported the frequency of symptoms in the studied population |
- Commentaries or opinions - Systematic or narrative reviews - Animal or biomechanical studies - Studies with <15 patients - Studies without an acceptable control group |
Population
This review targeted at studies on adult patients (>18 years) with cervical myelopathy secondary to spondylosis, disc herniation, ossification of the posterior longitudinal ligament (OPLL), congenital stenosis or subluxation. Eligible studies consisted of patients treated surgically or managed conservatively. Studies were excluded if they included patients with traumatic spinal cord injury, thoracic or lumbar myelopathy, tumor or infection.
Clinical Symptoms
Studies were included if they assessed the diagnostic accuracy or reported the frequency of clinical symptoms in DCM. Symptoms of interest included, but were not limited to, hand numbness, loss of dexterity, arm paresthesias, gait impairment, weakness, neck pain and bladder or bowel dysfunction. Studies were excluded if they only discussed clinical signs, patient- or clinician-reported outcome measures or imaging characteristics.
Outcome
Studies were included if they summarized the sensitivity, specificity, positive or negative predictive value or positive or negative likelihood ratio of a symptom. In some cases, sensitivity was calculated from the frequency of a clinical sign in a DCM population.
Study Design
For KQ1, this review targeted cohort, case-control or case-based studies that included an acceptable control group for comparison. An example of an appropriate control group is a group of individuals with cervical radiculopathy or axial neck pain with no evidence of myelopathy or cord compression. For KQ2, this review identified clinical studies that reported the frequency of various symptoms in patients with DCM. Studies were excluded if they were commentaries or opinions, systematic or narrative reviews, animal or biomechanical studies or consisted of less than 15 participants (patients or healthy controls).
Search, Study Selection and Data Collection Process
In Davies et al (2018) established and validated a highly sensitive MEDLINE search filter for DCM in order to optimize literature reviews.15,16 Using this filter, a database was developed that includes all primary studies on DCM. For this scoping review, this database was accessed to identify all DCM papers published between 2005 and 2020. Only studies involving humans and written in English were considered for inclusion. Full text investigation of each study in the database was deemed necessary as the frequency of clinical symptoms of DCM may be reported in the methods or results section without being referred to in the abstract. The following data were extracted from each article: patient sample and characteristics, including diagnosis and treatment; relevant symptoms; and results on frequency and diagnostic accuracy.
Risk of Bias in Individual Studies
Risk of bias was not assessed given this was a scoping review and not a systematic review. 14 Furthermore, studies were not excluded based on risk of bias given the known paucity and heterogeneity of the evidence base.
Data Analysis
Forest plots were created using RevMan. From each article, we extracted the number of patients who had the disease and tested positive (true positive), did not have the disease and tested positive (false positive), had the disease and tested negative (false negative), and did not have the disease and tested negative (true negative). From these values, sensitivity and specificity were computed and plotted. In some studies, we estimated each value using prevalence data in combination with reported sensitivity and specificity. In other studies, only true positives were reported. The 95% confidence intervals for sensitivity and specificity were automatically generated by RevMan using standard error.
Results
Study Selection
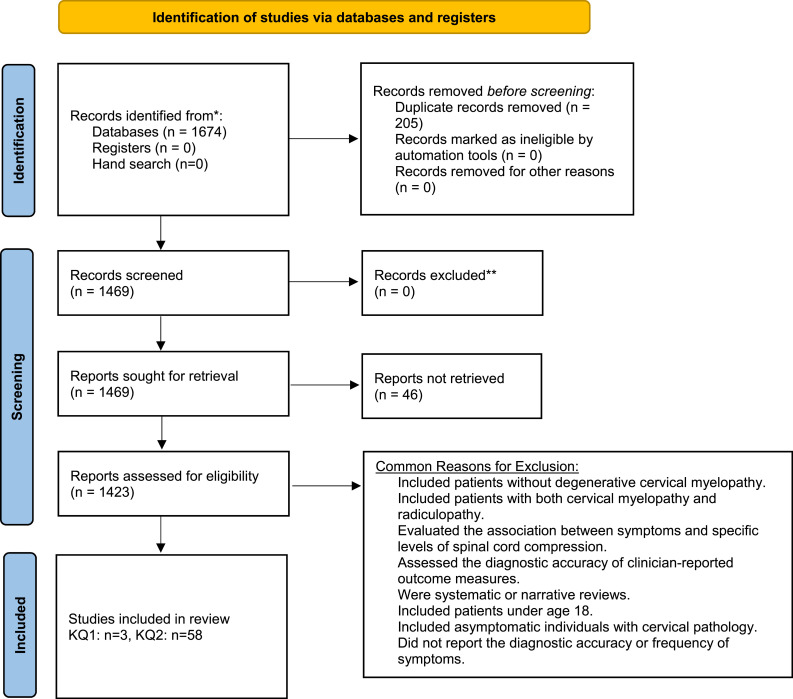
The search yielded a total of 1674 citations. Two-hundred and five duplicate studies were removed. The full text of 46 studies could not be located. After full text review, 1361 records were excluded. Three studies explored the diagnostic accuracy of common symptoms of DCM using an appropriate control group.17-19 An additional 58 studies reported on frequency of clinical signs in a cohort of DCM patients and were also included.6,19-75 Commonly, studies were excluded if they (i) discussed cervical spine pathology in asymptomatic individuals; (ii) included patients with both myelopathy and radiculopathy or myelopathy secondary to trauma, tumor or infection; (iii) were systematic or narrative reviews, surveys, posters or editorials; (iv) assessed the diagnostic accuracy of patient- or clinician-reported outcome measures; (v) had fewer than 15 patients; and (vi) were based on animal or computational models (Figure 1).
Figure 1.
An overview of the search process.
Study Characteristics
For KQ1, the search identified three studies that discussed the diagnostic accuracy of various symptoms and included a control group (Tables 2 and 3, Figure 2).17-19 Sample sizes ranged from 33 to 100. The most commonly reported symptom was neck pain (n = 2).18,19 All other symptoms were reported by single studies. Control groups included patients with signs and symptoms of early cervical myelopathy or cervical spine pain without evidence of cord compression or T2-signal change on MRI. For KQ2, an additional 58 studies were identified that reported on the frequency of symptoms in a cohort of patients with DCM (Table 4, Figures 3-8).6,19-74,76
Table 2.
Summary of Studies That Included a Control Group and Assessed the Diagnostic Accuracy of Various Clinical Symptoms.
| Author (Year), Study Design | Objective | Cervical Myelopathy and Control Group | Demographic Information | Symptoms | Metrics of Diagnostic Accuracy Assessed |
|---|---|---|---|---|---|
| Cheung et al (2018) | To translate and cross-culturally adapt the JOACMEQ into Traditional Chinese and to assess its validity, reliability and sensitivity for differentiating cervical myelopathy and presence of acute neck/shoulder pain | Cervical myelopathy group (n = 63) - Patients with cervical myelopathy secondary to CSM, OPLL and cervical subluxation or dislocation Control group (n = 37) - Patients without cervical myelopathy |
Age: 58.5 ± 12.2 Men: 60.0% |
Neck/shoulder pain | Sensitivity Specificity Positive and negative predictive value Positive and negative likelihood ratio |
| Cook et al (2009), prospective | To assess reliability and diagnostic accuracy of neurological tests and subjective findings associated with cervical myelopathy. | Cervical myelopathy group (n = 18) - Primary complaint of cervical spine pain with signal intensity changes on MRI confirming the presence of myelomalacia Control group (n = 27) - Primary complaint of cervical spine pain without MRI evidence of myelomalacia |
Age: 52 ± 13.4 Men: 41% |
Neck pain Loss of dexterity Hand Humbness Gait clumsiness |
Sensitivity Specificity Positive and negative predictive value Positive and negative likelihood ratio |
| Hori et al (2012) | To use novel diffusion metrics to estimate spinal cord compression in patients with early cervical spondylosis. | Cervical myelopathy group (n = 18) - Patients with signs and symptoms of cervical myelopathy and with evidence of spinal cord compression Control group (n = 15) - Patients with signs and symptoms of cervical myelopathy but without spinal cord compression |
Cervical myelopathy Age: 63.3 ± 10.8 Men: 33% Control Age: 50.5 ± 16.2 Men: 47% |
Numbness Pain Cervical vertigo Neck stiffness Hypalgesia Tremor Apraxia Jitteriness |
Sensitivity Specificity Positive and negative predictive value Positive and negative likelihood ratio |
Table 3.
Diagnostic Accuracy of Symptoms in Degenerative Cervical Myelopathy: Results of Three Studies That Included a Control Group.
| Clinical Symptom | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Positive Likelihood Ratio | Negative Likelihood Ratio |
|---|---|---|---|---|---|---|
| Gait clumsiness | ||||||
| Cook et al (2009) | 56 | 52 | 43 | 54 | 1.15 | .86 |
| Loss of dexterity | ||||||
| Cook et al (2009) | 72 | 26 | 39 | 58 | .98 | 1.07 |
| Hand numbness | ||||||
| Cook et al (2009) | 56 | 67 | 53 | 69 | 1.67 | .67 |
| Neck pain | ||||||
| Cheung et al (2018) | 76 | 11 | 59 | 21 | .85 | 2.20 |
| Cook et al (2009) | 94 | 18 | 44 | 83 | 1.16 | .30 |
| Numbness | ||||||
| Hori et al (2012) | 61 | 60 | 65 | 56 | 1.53 | .65 |
| Pain | ||||||
| Hori et al (2012) | 61 | 60 | 65 | 56 | 1.53 | .65 |
| Cervical vertigo | ||||||
| Hori et al (2012) | 6 | 87 | 33 | 43 | .42 | 1.09 |
| Neck stiffness | ||||||
| Hori et al (2012) | 11 | 87 | 50 | 45 | .83 | 1.02 |
| Hypalgesia | ||||||
| Hori et al (2012) | 6 | 87 | 33 | 43 | .42 | 1.09 |
| Tremor | ||||||
| Hori et al (2012) | 11 | 93 | 67 | 47 | 1.67 | .95 |
| Jitteriness | ||||||
| Hori et al (2012) | 0 | 93 | 0 | 44 | .00 | 1.07 |
| Apraxia | ||||||
| Hori et al (2012) | 6 | 93 | 50 | 45 | .83 | 1.01 |
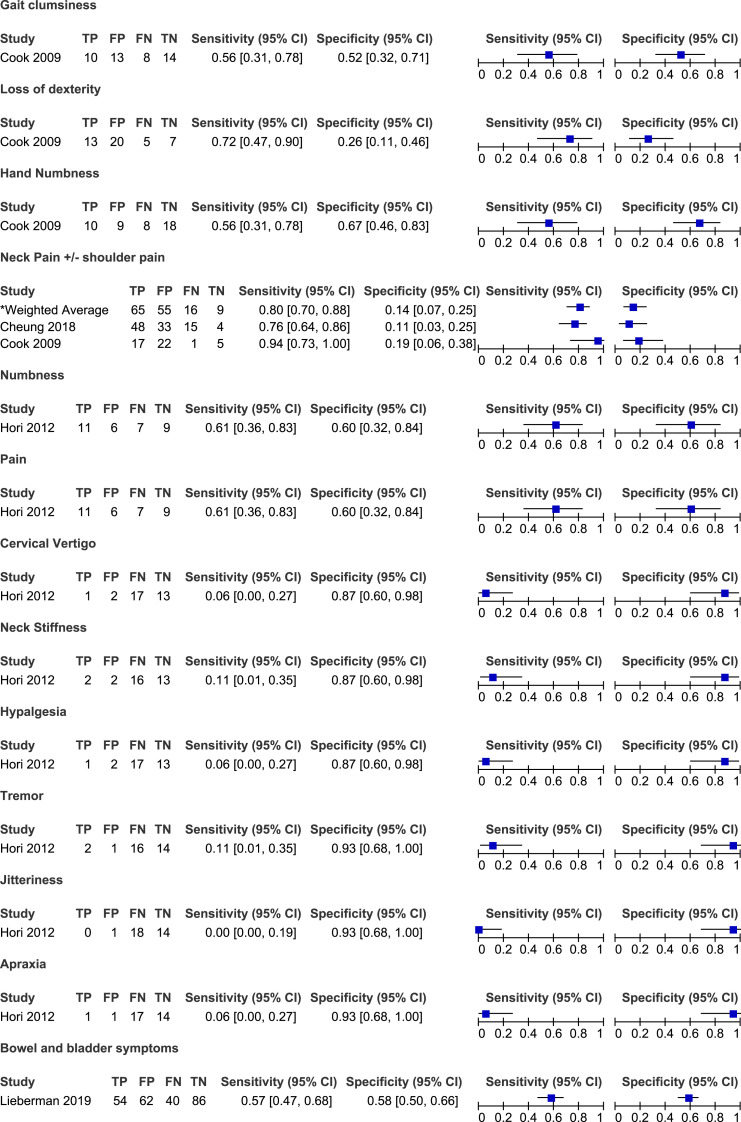
Figure 2.
Sensitivity and specificity of symptoms in degenerative cervical myelopathy: Results of three studies that included a control group.
Table 4.
Frequency of Symptoms in Patients With Degenerative Cervical Myelopathy.
| Clinical Symptom | Sensitivity | Clinical Symptom | Sensitivity |
|---|---|---|---|
| Autonomic symptoms | |||
| Bowel/bladder complaints | Sexual dysfunction | ||
| Ahmed et al (2020) | Sphincter disturbance: 43% | Chibbaro et al (2009) | 6% |
| Audat et al (2018) | 16% | He et al (2006) | 3% |
| Burkhardt et al (2017) | Bladder: 4% | Sinha and Jagetia (2011) | 6% |
| Chacko et al (2012) | Bladder: 33% | ||
| Chacko et al (2014) | Bladder: 42% | ||
| Chatley et al (2009) | Bladder: 42%, bowel: 33% | ||
| Chibbaro et al (2009) | 13% | ||
| El-Ghandour et al (2020) | Bladder: 42% | ||
| Holly et al (2009) | 10% | ||
| Holly et al (2017) | 13% | ||
| Hossam et al (2013) | Sphincter disturbance: 100% | ||
| Jain et al (2009) | 30% | ||
| Kang et al (2020) | 9% | ||
| Kim et al (2007) | Sphincter disturbance: 19% | ||
| Kim et al (2010) | Bladder: 17%, bowel: 6% | ||
| Kim et al (2018) | Bladder: 13% | ||
| Kommu et al (2014) | Sphincter disturbance: 21% | ||
| Lo (2007) | Sphincter disturbance: 7% | ||
| Misawa et al (2005) | Bladder: 52% | ||
| Moussellard et al (2014) | Bladder: 58% | ||
| Niu et al (2020) | Sphincter disturbance: CC: 1%, OS 17% | ||
| Raslan et al (2014) | Sphincter disturbance: 10% | ||
| Revanapa et al (2017) | Bladder: 68% | ||
| Scholler et al (2020) | 5% | ||
| Sinha and Jagetia (2011) | 12% | ||
| Turel et al (2013) | Bladder: 21% | ||
| Williams et al (2009) | Bladder: 17% | ||
| Zhang et al (2018) | 21% | ||
| Gait dysfunction and imbalance | |||
| Gait dysfunction | Difficulty climbing | ||
| Burkhardt et al (2017) | 87% | Thakar et al (2009) | 70% |
| Chatley et al (2009) | 86% | ||
| Chibbaro et al (2009) | 76% | ||
| Choi and Kim (2018) | 12% | ||
| Cui et al (2015) | 35% | ||
| Dong et al (2018) | 100% | ||
| Du et al (2013) | 57% | ||
| Holly et al (2009) | 76% | ||
| Holly et al (2017) | 69% | ||
| Hossam et al (2013) | 100% | ||
| Hou et al (2020) | 53% | ||
| Kim et al (2007) | 77% | ||
| Kong et al (2019) | 25% | ||
| Konya et al (2009) | 10% | ||
| Moussellard et al (2014) | 79% | ||
| Tetreault et al (2015) | 77% | ||
| Turel et al (2013) | 100% | ||
| Vitzthum and Dalitz (2007) | 86% | ||
| Wei et al (2019) | 55% | ||
| Williams et al (2009) | 83% | ||
| Zhang et al (2018) | 72% | ||
| Zhou et al (2015) | 42% | ||
| Zhou et al (2018) | 56% | ||
| Walking fatigue | Imbalance | ||
| Cole et al (2020) | 12% | ||
| Wang et al (2012) | 40% | Cui et al (2015) | 4% |
| Zhang et al (2018) | Gait: 25% | ||
| Pain symptoms | |||
| Radicular/Radiating pain | Neck and/or shoulder pain | ||
| Audat et al (2018) | 96% | Audat et al (2018) | 98% |
| Chacko et al (2012) | 14% | Burkhardt et al (2017) | 83% |
| Chatley et al (2009) | 39% | ||
| Choi and Kim (2018) | Neck or shoulder: 24% | ||
| Cole et al (2020) | 33% | ||
| Cui et al (2015) | 9% | ||
| Dong et al (2018) | Neck and shoulder: 100% | ||
| El-Ghandour et al (2020) | 75% | ||
| Gembruch et al (2019) | Neck and arm: 40% | ||
| Gerling et al (2017) | 51% | ||
| Jain et al (2009) | 100% | ||
| Kim et al (2010) | 50% | ||
| Kim et al (2018) | 19% | ||
| Chacko et al (2014) | 15% | ||
| Chatley et al (2009) | 27% | ||
| Cole et al (2020) | 9% | ||
| Kiris and Kilincer (2008) | 38% | ||
| Kong et al (2019) | 100% | ||
| Konya et al (2009) | Neck and arm: 90% | ||
| Lau et al (2017) | 66% | ||
| Lo (2007) | 35% | ||
| El-Ghandour et al (2020) | 51% | Moussellard et al (2014) | 52% |
| Niu et al (2020) | CC: 33%, OS 55% | ||
| Scholler et al (2020) | 75% | ||
| Thakar et al (2009) | 44% | ||
| Thakar and Rajshekhar (2012) | 43% | ||
| Kim et al (2007) | 35% | Williams et al (2009) | 54% |
| Zhang et al (2018) | 21% | ||
| Kiris and Kilincer (2008) | 20% (radicular symptoms) | ||
| Zhou et al (2015) | Neck or shoulder: 39% | ||
| Raslan et al (2014) | 38% | ||
| Scholler et al (2020) | 50% | Zhou et al (2018) | Neck or shoulder: 72% |
| Turel et al (2013) | 7% | ||
| Williams et al (2009) | 79% | ||
| Upper limb pain | Axial pain | ||
| Chibbaro et al (2009) | 26% | Cho et al (2010) | 100% |
| Gerling et al (2017) | 45% | Hossam et al (2013) | 87% |
| Konya et al (2009) | 10% | Kato et al (2008) | 19% |
| Niu et al (2020) | CS: 37%, OS: 54% | Thakar and Rajshekhar (2012) | 24% |
| Thakar and Rajshekhar (2012) | 51% | Zhang et al (2020) | 54% |
| Back pain | Lower limb pain | ||
| Cole et al (2020) | 9% | Konya et al (2009) | 10% |
| Kawakita et al (2009) | Lower: 22% | ||
| Unspecified pain | Funicular pain | ||
| Chiu and Pang (2017) | 24% | Chatley et al (2009) | 5% |
| Vitzthum and Dalitz (2007) | 67% | ||
| Headache | Chest/Abdominal discomfort | ||
| Williams et al (2009) | 8% | Kim et al (2007) | 58% |
| Spasm | |||
| Konya et al (2009) | Paravertebral: 90% | ||
| Motor symptoms | |||
| Hand clumsiness | Loss of hand Function/Fine motor disturbance | ||
| Chatley et al (2009) | 73% | ||
| Holly et al (2009) | 71% | ||
| Chibbaro et al (2009) | 56% | Holly et al (2017) | 63% |
| Choi and Kim (2018) | 47% | Wang et al (2012) | 53% |
| Cui et al (2015) | 26% | Williams et al (2009) | 33% |
| Zhang et al (2018) | 22% | ||
| Hossam et al (2013) | 90% | ||
| Hou et al (2020) | 67% | ||
| Lo (2007) | 72% | ||
| Tetreault et al (2015) | 75% | ||
| Thakar et al (2009) | 80% | ||
| Wei et al (2019) | 55% | ||
| Any hand symptom (pain, numbness, weakness or loss of dexterity) | Any upper extremity symptom (weakness, sensory loss or loss of dexterity) | ||
| Zhou et al (2015) | 68% | ||
| Cole et al (2020) | 85% | ||
| Zhou et al (2018) | 61% | ||
| Difficulty eating | Unspecified fine motor difficulties | ||
| Thakar et al (2009) | 54% | Gerling et al (2017) | 28% |
| Hand weakness | Foot weakness | ||
| Choi and Kim (2018) | 18% | Konya et al (2009) | 10% |
| Cui et al (2015) | 4% | ||
| Upper extremity clumsiness | Lower extremity clumsiness | ||
| Cui et al (2015) | 4% | Cui et al (2015) | 4% |
| Upper extremity weakness | Lower extremity weakness | ||
| Ahmed et al (2020) | 63% | ||
| Ahmed et al (2020) | 87% | Chatley et al (2009) | 3% |
| Chatley et al (2009) | 16% | Cui et al (2015) | 4% |
| Chibbaro et al (2009) | 66% | Du et al (2013) | 29% |
| Choi and Kim (2018) | 47% | Kim et al (2018) | 41% |
| Cole et al (2020) | 12% | Konya et al (2009) | 10% |
| Niu et al (2020) | CC: 29%, OS: 81% | ||
| Cui et al (2015) | 4% | Raslan et al (2014) | 33% |
| Du et al (2013) | 58% | Williams et al (2009) | 88% |
| Zhang et al (2018) | 43% | ||
| Kim et al (2018) | 19% | ||
| Niu et al (2020) | CC: 34%, OS: 83% | ||
| Raslan et al (2014) | 43% | ||
| Williams et al (2009) | 92% | ||
| Zhang et al (2018) | 38* | ||
| Weakness | |||
| Asher et al (2019) | 55% | ||
| Burkhardt et al (2017) | 57% | ||
| Chatley et al (2009) | Mean: 78%, all 4 limbs: 48% | ||
| El-Ghandour et al (2020) | 91% | ||
| Kommu et al (2014) | 97% | ||
| Kong et al (2019) | 50% | ||
| Rajashekaran et al (2016) | 23% | ||
| Scholler et al (2020) | 15% | ||
| Tetreault et al (2015) | 83% | ||
| Wei et al (2019) | 73% | ||
| Sensory symptoms | |||
| Upper extremity numbness | Lower extremity numbness | ||
| Chiu and Pang (2017) | 44% | Cui et al (2015) | 17% |
| Lo (2007) | 65% | ||
| Choi and Kim (2018) | 6% | ||
| Thakar et al (2009) | 91% | ||
| Cui et al (2015) | 4% | ||
| Williams et al (2009) | 83% | ||
| Konya et al (2009) | 60% | ||
| Zhang et al (2018) | 57% | ||
| Lo (2007) | 83% | ||
| Thakar et al (2009) | Hands and arms: 96% | ||
| Zhang et al (2018) | 74% | ||
| Hand numbness | Unspecified numbness | ||
| Cole et al (2020) | 21% | Ahmed et al (2020) | 73% |
| Asher et al (2019) | 37% | ||
| Cui et al (2015) | 52% | Chiu and Pang (2017) | 100% |
| Kim et al (2007) | 42% | Dong et al (2018) | 100% |
| Tetreault et al (2015) | 89% | Du et al (2013) | 51% |
| Kong et al (2019) | 88% | ||
| Wang et al (2012) | 53% | Rajasekaran et al (2016) | 57% |
| Raslan et al (2014) | 62% | ||
| Wei et al (2019) | 87% | ||
| Great toe numbness | Trunk numbness | ||
| Cui et al (2015) | 4% | ||
| Zhang et al (2018) | 5% | ||
| Cui et al (2015) | 4% | ||
| Unspecified upper extremity sensory symptoms | Unspecified lower extremity sensory symptoms | ||
| Chibbaro et al (2009) | 40% | ||
| Holly et al (2009) | 29% | ||
| Chibbaro et al (2009) | 78% | ||
| Kim et al (2018) | 25% | ||
| Kim et al (2018) | 75% | ||
| Niu et al (2020) | OS: 17% | ||
| Niu et al (2020) | CC: 46%, OS: 71% | ||
| Unspecified hand sensory symptoms (numbness or paresthesias) | Sensory radicular symptoms | ||
| Jain et al (2009) | 100% | ||
| Holly et al (2009) | 48% | ||
| Holly et al (2017) | 44% | ||
| Hossam et al (2013) | 100% | ||
| Hand paresthesias | Upper extremity paresthesia | ||
| Choi and Kim (2018) | 24% | Choi and Kim (2018) | 29% |
| Tetreault et al (2015) | 57% | ||
| Vitzthum and Dalitz (2009) | 70% | ||
| Moussellard et al (2014) | 93% | ||
| Wei et al (2019) | 54% | ||
| Williams et al (2009) | 67% | ||
| Lower extremity paresthesia | Unspecified paresthesias | ||
| Chacko et al (2012) | 86% | ||
| Chacko et al (2014) | 85% | ||
| Williams et al (2009) | 58% | ||
| Kim et al (2010) | 92% | ||
| Turel et al (2013) | 86% | ||
| Sensory change or disturbance | Tightness of the trunk or legs | ||
| Hossam et al (2013) | 53% | ||
| Burkhardt et al (2017) | 78% | ||
| Kang et al (2020) | 53% | ||
| Kim et al (2010) | 36% | ||
| Scholler et al (2020) | 90% | ||
| Zonesthesia/Band-like sensation at chest or trunk | Heaviness | ||
| Dong et al (2018) | 100% | ||
| Ahmed et al (2020) | 83% | ||
| Wang et al (2012) | 38% | ||
| Zhang et al (2018) | 20% | ||
| Other | |||
| Dyskinesia | Respiratory difficulty | ||
| Chatley et al (2009) | 2% | ||
| Kang et al (2020) | 37% | ||
| Moussellard et al (2014) | 0% | ||
| L’hermitte phenomenon | Knee buckling | ||
| Kiris and Kilincer (2008) | 88% | ||
| Chatley et al (2009) | 2% | ||
| Hossam et al (2013) | 77% | ||
| Tetreault et al (2015) | 27% | ||
| Wei et al (2019) | 19% | ||
| Giddiness | Dizziness | ||
| Audat et al (2018) | 54% | ||
| Williams et al (2009) | 8% | ||
| Sugawara et al (2009) | 95%; vertigo: 10%, disequilibrium: 29%, presyncope: 0%; light-headedness: 86% | ||
| Stiffness | |||
| Chatley et al (2009) | 80% | ||
| Hossam et al (2013) | 60% | ||
| Lo (2007) | Leg: 61% | ||
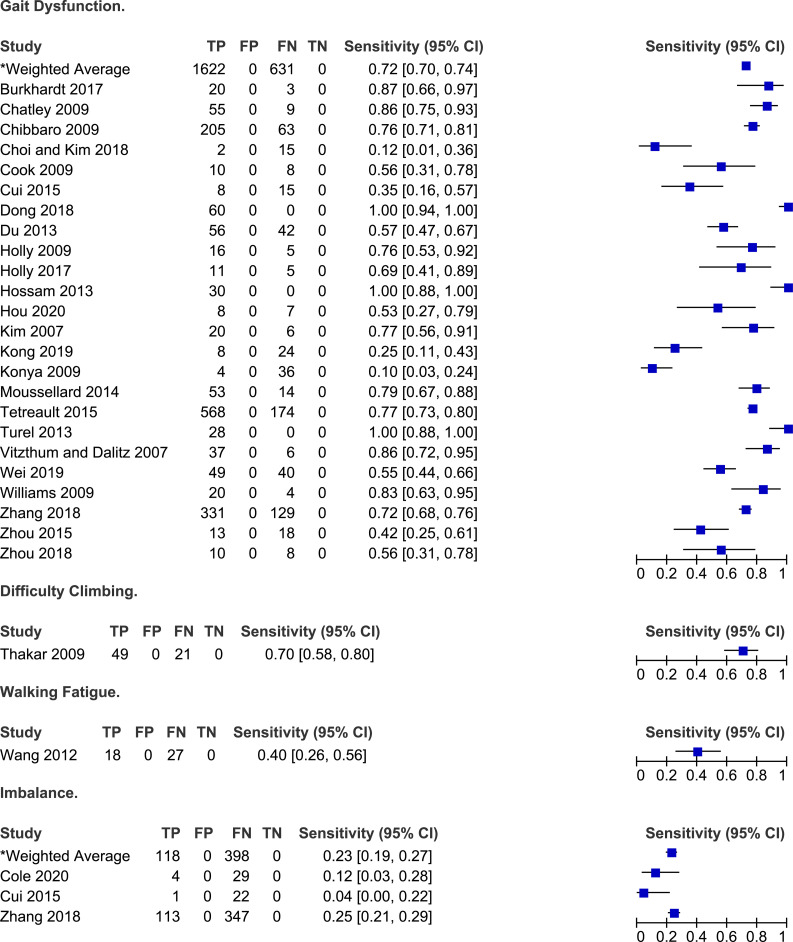
Figure 3.
Frequency of gait impairment and imbalance in degenerative cervical myelopathy.
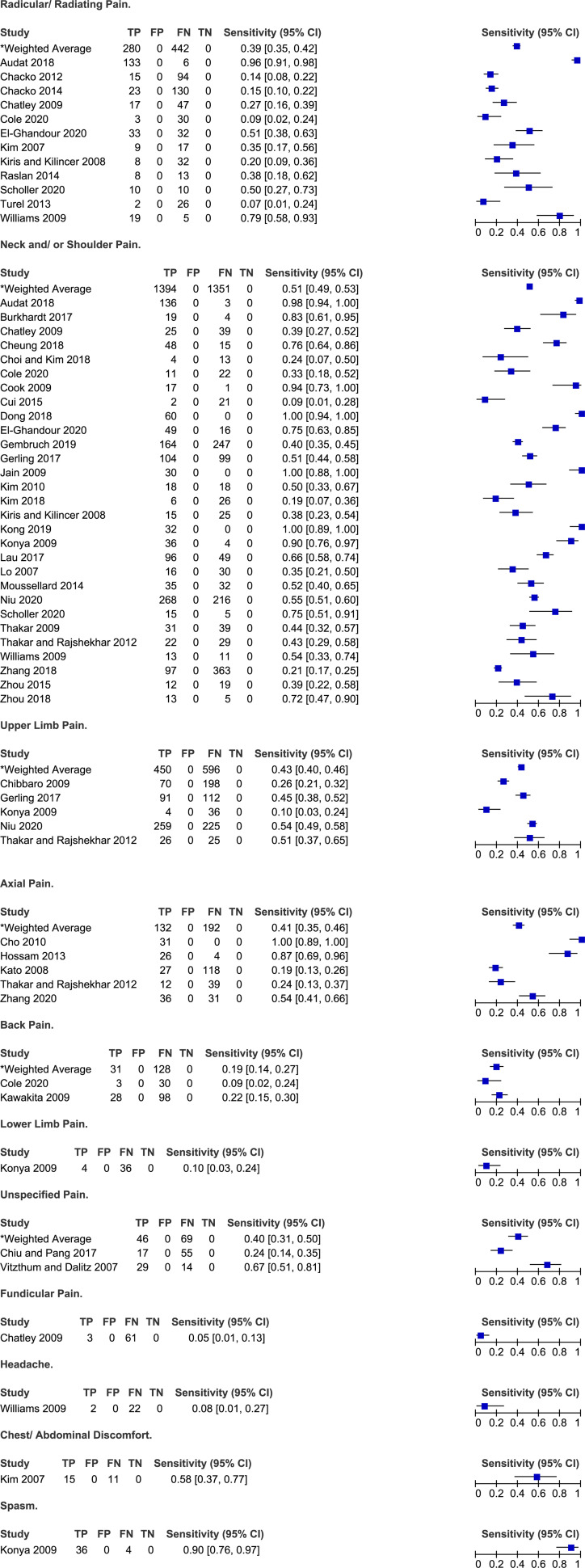
Figure 4.
Frequency of pain symptoms in degenerative cervical myelopathy.
Figure 5.
Frequency of motor symptoms in degenerative cervical myelopathy.
Figure 6.
Frequency of sensory symptoms in degenerative cervical myelopathy.
Figure 7.
Frequency of autonomic symptoms in degenerative cervical myelopathy.
Figure 8.
Frequency of other symptoms reported by patients with degenerative cervical myelopathy.
Results of Individual Studies
Studies That Included a Control Group
Cook et al (2009) compared the frequency of various symptoms between patients with hyperintensity on T2-weighted MRI and those without signal change. Based on their results, the most sensitive symptom for diagnosing myelopathy was current neck pain (94%), followed by loss of dexterity (72%), numbness in hands (56%) and clumsiness during gait (56%). 19 In contrast, neck pain (18%) and loss of dexterity (26%) had poor specificity. Finally, based on likelihood ratios, none of these four stand-alone symptoms demonstrated the ability to influence post-test probability with a positive or a negative finding. Cheung et al 18 (2018) evaluated the frequency of neck or shoulder pain in patients with and without DCM. Based on their results, the sensitivity of neck pain for diagnosing cervical myelopathy was 76% and the specificity was 11%. Finally, Hori et al 17 (2012) separated patients with clinical signs and symptoms of early cervical myelopathy into two groups based on whether their spinal cord was compressed or not on MRI. The presence of numbness and pain was moderately sensitive (61%) and specific (60%) for diagnosing DCM. All other symptoms had low sensitivity but high specificity, including cervical vertigo (6%, 87%), neck stiffness (11%, 87%), hyperalgesia (6%, 87%), tremor (11%, 93%), jitteriness (0%, 93%) and apraxia (6%, 93%) (Tables 2 and 3, Figure 2)
Studies That Reported on the Frequency of Symptoms in Patients With Degenerative Cervical Myelopathy
Symptoms Related to Gait
Twenty-three studies discussed the frequency of gait dysfunction in patients with DCM.6,23,25,26,28,30,32,33,38-41,48,51,52,56,66-68,70,71,73,74 Based on their results, the sensitivity of gait impairment for diagnosing DCM ranged from 10% to 100% with a weighted average of 72% (95% CI 70%-74%). In three studies, all patients reported a degree of gait instability or dysfunction.32,40,66 In single studies, the frequency of walking fatigue and difficulty climbing was 40% and 70%, respectively.64,69 Finally, rates of imbalance ranged from 4% to 25%.30,31,71
Symptoms Related to Pain
Several studies reported on the frequency of various types of pain in patients with DCM: radicular or radiating (n = 12),20,24,25,31,34,48,49,59,61,66,67,75 neck and/or shoulder (n = 27),20,23,25,28,30-32,34-36,42,46,47,49,51-54,56,57,61,64,65,67,71,73,74 upper extremity (n = 5),26,36,52,57,65 axial (n = 5),27,40,44,65,72 back (n = 2)31,45 or unspecified (n = 2) pain.29,68 Furthermore, single studies presented the incidence of lower extremity pain, 52 funicular pain, 25 chest and/or abdominal discomfort 48 and headache. 67 A study by Niu et al 57 (2020) aimed to summarize the primary complaints as well as other symptoms experienced by patients undergoing surgery for DCM. Based on their results, neck pain was the chief complaint in 33% of patients, while upper extremity pain was the chief complaint in 37%. Interestingly, upper extremity pain was more common when the level of maximal spinal cord compression was more distal in the cervical spine. The frequency of different types of pan varied significantly across studies: (i) 7%-93% for radicular pain (weighted average: 39%, 95% CI 35%-42%); (ii) 9%-100% for neck and/or shoulder pain (weighted average: 51%, 95% CI 49%-53%); (iii) 10%-54% for upper extremity pain (weighted average: 43%, 95% CI 40%-46%); and (iv) 19%-100% for axial pain (pain extending from the nuchal to scapular regional, weighted average 41%, 95% CI 35%-46%). Only a minority of patients with DCM reported headache (8%), lower extremity pain (10%) or back pain (9-22%).
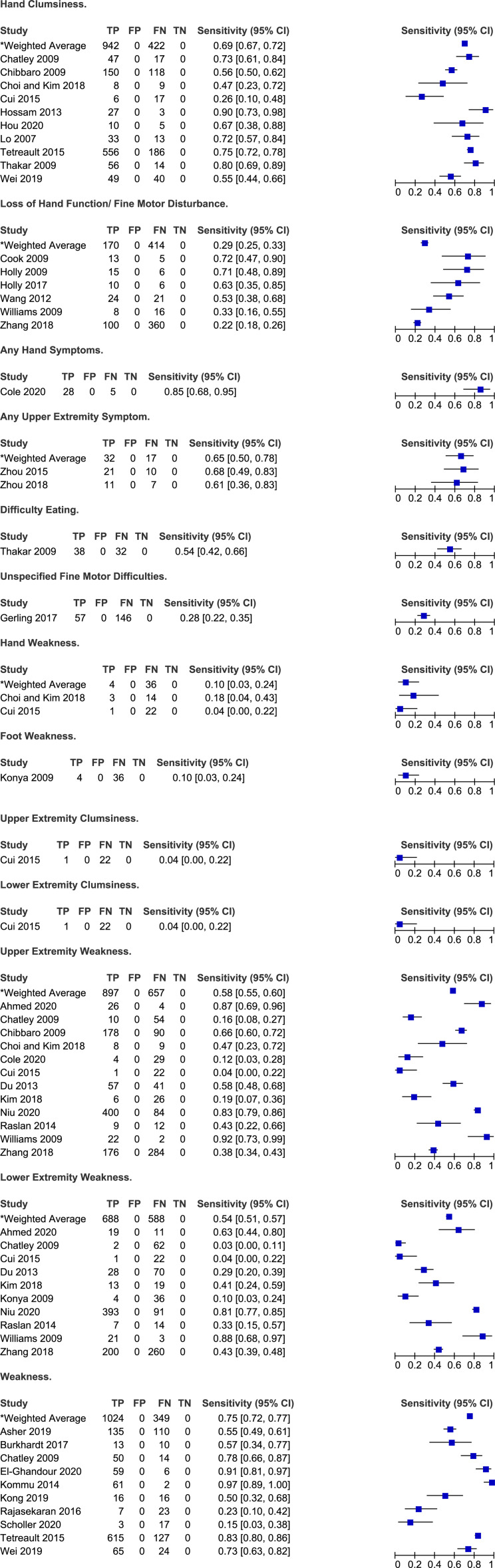
Symptoms Related to Hand and Upper and Lower Extremity Motor Function
Several studies reported on the frequency of symptoms related to hand function in patients with DCM. Rates of hand clumsiness ranged from 26% to 90% across ten studies with a weighted average of 69% (95% CI 67%-72%).6,25,26,28,40,41,54,64,70 Hand function was also described in terms of loss of dexterity and fine motor disturbance in five studies.38,39,67,69,71 In two studies by Holly et al (2009, 2017), 63%-71% of patients reported significant changes in their ability to use utensils, sew, write or do up buttons.38,39 Similarly, Thakar et al 64 (2009) determined that 54% of patients experience difficulty eating, potentially due to difficulty manipulating a fork and knife. The frequency of deterioration in hand function was lower in three other studies and ranged from 22% to 53%.67,69,71 Cole et al 31 (2020) reported 85% of patients with DCM had at least one hand symptom, either pain, numbness, weakness or loss of dexterity. Finally, across two studies, the frequency of hand weakness ranged from 4% to 18%.28,30
Twelve studies discussed the frequency of upper extremity motor symptoms.21,25,26,28,30,31,33,47,57,59,67,71 In a single study by Cui et al 30 (2015), clumsiness of the upper limb was much less frequent (4%) among patients with DCM than hand clumsiness (26%). The frequency of upper extremity weakness ranged from 4% to 92% across 12 studies with a weighted average of 58% (95% CI 55%-60%). In Niu et al 57 (2020), while upper extremity weakness was present in 83% of patients with DCM, it was the chief complaint in only 34%.
Similarly, ten studies reported rates of lower extremity motor symptoms in patients with DCM.21,25,30,33,47,52,57,59,67,71 Rates of lower extremity weakness ranged from 3% to 88% with a weighted average of 54% (95% CI 51%-57%). An additional ten studies were identified that did not distinguish between weakness affecting the upper and lower extremities.6,22,23,25,34,50,51,58,61,70 Across these studies, the frequency of weakness ranged from 15% to 97% with a weighted average of 75% (95% CI 72%-77%).
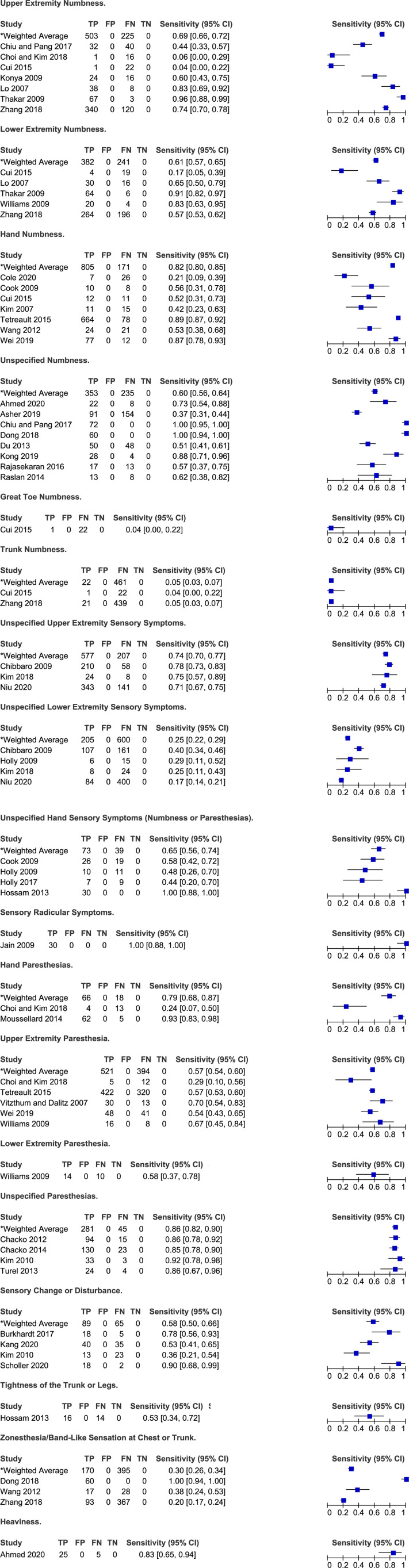
Symptoms Related to Hand and Upper and Lower Extremity Sensory Function
Eleven studies reported on the frequency of sensory hand complaints in patients with DCM: numbness (n = 6),6,30,31,48,69,70 paresthesias (=2)28,56 and unspecified (numbness or paresthesias, n = 3).38-40 Based on their results, the frequency of hand numbness ranged from 21% to 89% (weighted average: 82%, 95% CI 80%-85%) while the frequency of hand paresthesias ranged from 24% to 93% (weighted average: 79%, 95% CI 68%-87%). In two studies by Holly et al (2009, 2017), 44%-48% of patients reported hand sensory symptoms, whereas in a third study by Hossam et al (2013), all patients had either hand numbness or paresthesias.38-40
Based on the results of seven studies, the sensitivity of upper extremity numbness for diagnosing DCM ranged from 4% to 96% with a weighted average of 69% (95% CI 66%-72%)28-30,52,54,64,71 Similarly, the frequency of upper extremity paresthesias ranged from 29% to 70% (weighted average 57%, 95% CI 54%-60%).6,28,67,68,70 In a study by Niu et al 57 (2020), the most common chief complaint was upper extremity sensory symptoms (46%); however, it was the third most common overall symptom (71%). Finally, any upper extremity sensory complaint was present in 71%-78% of patients with DCM.26,47
Nine studies discussed the frequency of lower extremity sensory symptoms in a DCM population: numbness (n = 5),30,54,64,67,71 paresthesias (n = 1) 67 and unspecified (n = 4).26,39,47,57 The sensitivity of lower extremity numbness ranged from 17% to 91% with a weighted mean of 61% (95% CI 57%-65%).30,54,64,67,71 In a single study by Williams et al 67 (2009), 58% of patients reported lower extremity paresthesias. Finally, rates of any lower extremity sensory symptom were lower than rates of any upper sensory symptom and ranged from 17% to 40% (weighted average: 25%, 95% CI 22%-29%).26,39,47,57
Based on two studies, a minority of patients complained of great toe (4%) and trunk numbness (4%-5%).30,71 Rates of zonesthesia (ie band-like sensation at chest or trunk) ranged from 20% to 100% across three studies.32,69,71 Finally, 53% of patients experienced tightness of trunk or legs and 83% reported heaviness.21,40 Rates of unspecified numbness varied from 37% to 100% across eight studies (weighted average: 60%, 95% CI 56%-64%),21,22,29,32,33,51,58,59 while rates of unspecified paresthesias ranged from 82% to 92% across four studies (weighted average: 86%, 95% CI 82%-90%).24,46,66,75
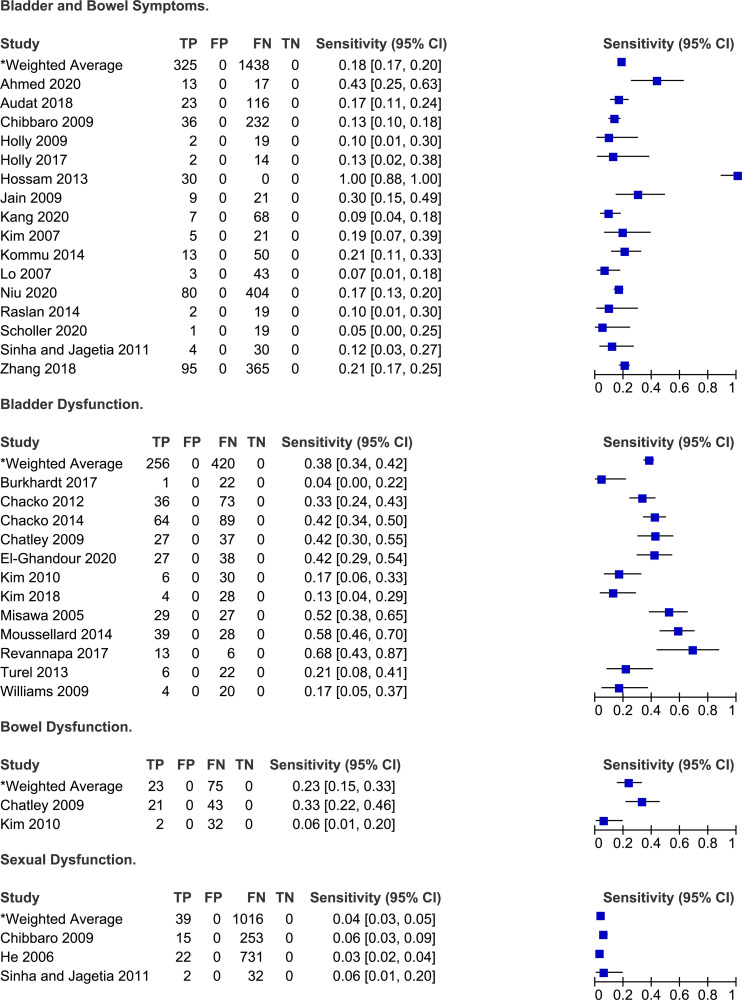
Symptoms Related to Autonomic Function
Twenty-eight studies reported the frequency of bladder and/or bowel dysfunction in patients with DCM.20,21,23-26,34,38-40,42,43,46-48,50,54-57,59-62,66,67,71,75 In a study by Misawa et al 55 (2008), patients were asked about subjective urinary symptoms and were required to complete a three day voiding diary. Based on their results, 16 (68%) patients reported difficulty urinating, 10 (48%) felt as though they had residual urine, five (24%) experienced urgency and one (5%) lacked a desire to void. Based on the three day voiding diary, two patients had urinary retention, four experienced nocturia, one had oliguria and four had episodes of urge incontinence. Across several studies, the sensitivities of bladder dysfunction for diagnosis DCM ranged from 4% to 68% with a weighted average of 38% (95% CI 34%-43%). Other studies did not distinguish between bladder and bowel dysfunction and either reported the frequency of sphincter disturbance (7%-100%)21,40,48,50,54,57,59 or of bladder and/or bowel dysfunction (5%-30%).20,26,38,39,42,43,61,62,71
Three studies reported the frequency of sexual dysfunction in a cohort of DCM patients.26,37,62 In He et al 37 (2006), patients were included in the “sexual dysfunction” group if they reported difficulty in penile erection or ejaculation. Based on their results, approximately 3% of patients undergoing surgery for DCM experienced sexual dysfunction. Of these, the majority (82%) had an abnormal psychogenic erection (ie erection resulting from extrinsic stimuli), while only 18% demonstrated an abnormal reflexive erection (ie erection elicited by direct penile stimulation). Across three studies, the sensitivity of sexual dysfunction for diagnosing DCM ranged from 3% to 6%.26,37,62
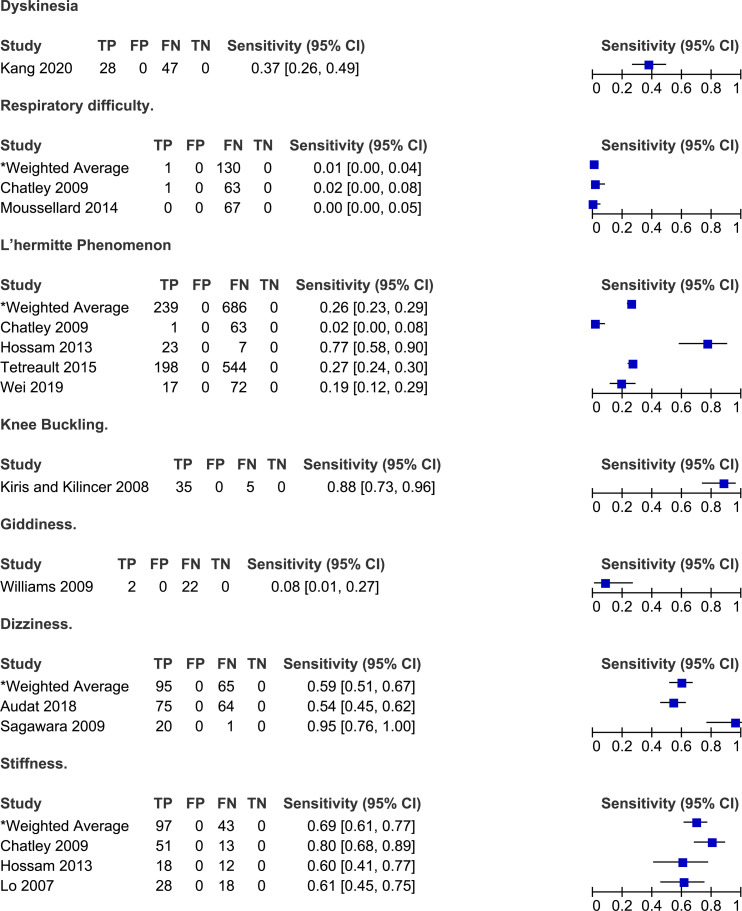
Other Symptoms
Studies have also reported the frequency of symptoms that do not fall into the above categories.6,20,25,40,43,49,54,56,63,67,70 Based on the results of four studies, 2%-77% of patients with DCM experience Lhermitte’s phenomena (weighted average: 25%, 95% CI 23%-29%).6,25,40,70 In a study by Sugawara et al (2009), 95% of patients with DCM reported episodes of dizziness, described as vertigo (10%), disequilibrium (29%) or light-headedness (86%). Finally, a minority of patients complained of respiratory difficulties (0%-2%), dyskinesias (37%) or giddiness (8%).25,43,56,67
Discussion
This scoping review aimed to summarize the diagnostic accuracy of various symptoms reported in patients with DCM. Unfortunately, there is a paucity of studies that compared the frequency of various symptoms between patients with confirmed cervical myelopathy and a control group. Furthermore, the control group in the three studies that met inclusion criteria for KQ1 was either not well defined or was based on the absence of certain imaging findings (eg cord compression, hyperintensity on T2-weighted images). It may be difficult to distinguish patients with DCM from those without using MRI characteristics due to poor correlation between imaging findings and disease severity. 77 Based on the results of these three studies, the presence of neck pain is moderately to highly sensitive for diagnosing DCM, but not specific. While these findings carry face validity, many of the other results from KQ1 do not. For example, stand-alone findings of tremor, cervical vertigo, jitteriness and apraxia are probably not specific for DCM as they can be present in a wide range of neurologic disorders, including Parkinson’s disease, stroke, vestibular dysfunction and cerebellar pathology. It is important to note that the presented results for sensitivity and specificity in this review are extracted from studies that are screening a particular population (and not just a random group of individuals).
Symptoms Related to Gait
Patients with DCM may experience gait instability, walking fatigue or difficulty climbing up or down the stairs. The proportion of individuals with gait dysfunction ranged from 10% to 100%. It is postulated that gait impairment in DCM is a result of both upper motor neuron and proprioceptive dysfunction as well as damage to the rubrospinal, vestibulospinal and reticulospinal tracts.78,79 Patients with early DCM will often have subtle instability and difficulty maintaining posture whereas those with more severe disease also have a component of weakness and spasticity that contributes to gait impairment. Several studies have analyzed various gait parameters and have identified that patients with DCM tend to walk slower, have difficulties generating adequate stride length, and spend less time in single support.80-84 While not captured in this scoping review, DCM must also be considered in patients with recurrent falls. 85 Notably, many elderly patients with DCM may consider gait instability as a natural part of aging and therefore may not report it when asked about symptoms. Therefore, history taking should critically evaluate if gait disturbance developed in conjuncture with other myelopathy-related symptoms. Furthermore, some older patients may not be aware of their gait impairment due to frailty and reduced mobility from other medical conditions (eg, degenerative and inflammatory joint disease, peripheral neuropathy, nutrient deficiency). As such, a thorough physical examination including gait assessments is essential when evaluating an individual for potential DCM.
Symptoms Related to Pain
Patients with DCM can present with neck, shoulder, axial, radicular, or diffuse neuropathic pain. Axial or neck pain arises from changes in the musculoskeletal structures including the paraspinal muscles, ligaments or vertebral bodies, whereas radicular pain is secondary to irritation of the nerve roots as they exit the spinal canal. Presence of pain and stiffness can significantly affect a patient’s quality of life, disturb sleep and limit ability to perform activities. 86 Based on this scoping review, neck and/or shoulder pain was the most frequent type of pain, followed by upper extremity pain and axial pain. Of note, this review intended to exclude patients with cervical radiculopathy (unless used as a control group to explore other symptoms) or myeloradiculopathy; as such, the incidence of radiating pain is potentially even higher than reported. Finally, back and lower extremity pain are not common symptoms of DCM and may only be present if a patient has concomitant lumbar arthritis and stenosis. Not surprisingly, neck pain is not specific for DCM. In fact, approximately 30%-50% of adults will experience neck pain in any given year. 87 In addition, a primary care practitioner will, on average, assess seven patients per week with neck or upper extremity symptoms. 88 It is advised that patients with complaints of neck pain be questioned and examined for evidence of myelopathy.
Symptoms Related to Hand and Upper and Lower Extremity Motor Function
Hand symptoms are common in patients with DCM, including clumsiness, loss of dexterity and weakness. In fact, 85% of patients with DCM may exhibit at least one symptom involving their hands. 31 Based on the results of this scoping review, hand clumsiness is typically present in 69% of patients with DCM. Individuals with DCM will often report difficulties manipulating small objects such as buttons or screws, using utensils to eat or typing on a keyboard.38,39,64 Furthermore, patients may also complain that they often drop objects. Hand dysfunction in patients with DCM is due to a combination of increased stretch reflexes and worsening proprioceptive function from underlying injury of the corticospinal tracts and dorsal columns. 89 A detailed examination of the hand may unveil finger extensor and abductor weakness, inability to grip and release and loss of proprioception. 90 Upper and lower extremity motor dysfunction may also be present in patients with DCM; however, the weighted averages are less than that of hand clumsiness. In patients with otherwise unspecific symptoms, fine motor dysfunction should be considered a characteristic symptom in DCM.
Symptoms Related to Hand and Upper and Lower Extremity Sensory Function
Patients may complain of sensory disturbances including numbness or paresthesias of their hands and upper or lower extremities. Based on the results of this scoping review, there is no classical pattern or distribution of sensory symptoms in DCM. The weighted averages range from 60% to 82% for hand, upper extremity and lower extremity numbness and from 57% to 79% for similarly distributed paresthesias. Although patients often experience bilateral sensory symptoms due to extrinsic compression of the cord, DCM should not be ruled out in individuals with unilateral symptoms. Importantly, DCM is often misdiagnosed as carpal tunnel syndrome due to overlapping symptoms including paresthesias, hand wasting and loss of dexterity. Furthermore, as DCM, carpal tunnel syndrome is often present bilaterally with one side that might dominate. 91 Patients with suspected carpal tunnel syndrome must be asked targeted questions about other symptoms consistent with myelopathy as well as examined for corticospinal and sensory tract dysfunction. A coexistence of DCM and carpal tunnel syndrome is also possible. Thus, further electrodiagnostic evaluation might be critical in these patients.
Symptoms Related to Autonomic Function
Patients with DCM may report bladder, bowel or sexual dysfunction. A lesion in the cervical spinal cord sometimes manifests as a spastic bladder with symptoms of increased urinary frequency and incontinence due to detrusor-sphincter dyssynergia and impaired feedback from the pontine micturition center. 92 Furthermore, according to Misawa et al 55 (2008), patients with DCM had a variety of urinary symptoms, including difficulty urinating and inability to completely empty the bladder. Of the studies that separately reported on bowel dysfunction, the frequency of difficulties with defecation in patients with DCM was low (6%-33%) compared to urinary complaints (4%-68%). Finally, patients with DCM rarely complained of sexual dysfunction (3%-6%).26,37,62 In general, sexual dysfunction is often underreported by patients due to embarrassment or because of the perception that difficulties maintaining an erection or achieving an orgasm are natural parts of aging. 93 Furthermore, all of the included studies emphasized male sexual health and none reported the impact of DCM on female sexual function. Of note, patients with DCM were more likely to have an intact reflexogenic erection, thought to originate from the sacral segments of the spinal cord, than an intact psychogenic erection which arises from the cerebrum and is modulated through the thoracic and lumbar segments. 37 Other studies have confirmed that patients with complete upper motor neuron lesions experience difficulties obtaining a psychogenic compared to a reflexogenic erection. 94 It is critical that specific questions be asked about bladder, bowel and sexual function in patients suspected to have DCM as these symptoms may be underreported.
Clinical Implications and Future Directions
When evaluating an individual with suspected DCM, it is important to specifically ask about hand function and fine motor skills (eg tying up buttons, using a screwdriver, doing up jewellery), gait instability, falls, sensory symptoms (eg numbness or paresthesias), neck, shoulder or arm pain, and bladder, bowel or sexual dysfunction. Some patients with DCM will have a dominant and disabling symptom and may fail to report other issues unless directly asked. The presence of symptoms with moderate to high sensitivity for identifying DCM should trigger a clinician to order further neuroimaging to either confirm or rule out this diagnosis. This review also emphasizes that DCM may initially present with subtle, vague or unusual symptoms, indicating that physicians must carry a high degree of clinical suspicion to ensure that DCM is not missed.
This scoping review serves as a first step in identifying the symptoms that should be included in diagnostic criteria for DCM. This will allow clinicians, notably primary care physicians, to better identify DCM, pursue timely neuroimaging and not miss a diagnosis of DCM when it presents in an uncommon way. If patients with DCM can be detected earlier, then they can be referred to specialists with expertise in the treatment of this condition. Ongoing studies have indicated that timely diagnosis and management of DCM results in superior neurological and functional recovery as well as reduces unemployment, dependency on others and healthcare costs.
While this is the first review to summarize current evidence on symptoms in DCM, there are limitations that should be mentioned. First, there were only three studies that included a control group in their analysis; as a result, there is limited information on the specificity of various DCM symptoms. Furthermore, individuals were considered controls if they had cervical spine pain or signs/symptoms of myelopathy but did not have myelomalacia or spinal cord compression on neuroimaging. It is increasingly appreciated that patients can still be diagnosed with DCM in the absence of signal change or even spinal cord compression on static MRI; as such, these control groups may be suboptimal for assessing the accuracy of various symptoms. Second, values for sensitivity and specificity are extracted from studies that are screening a particular population and not just a random group of individuals. Further investigation is required to better calculate sensitivity and specificity of various symptoms using adequate control groups. Nonetheless, this review provides invaluable information on some of the most common symptoms of DCM and will undoubtedly improve understanding of this condition.
Conclusion
Patients with DCM can present with a wide variety of symptoms in their upper and lower extremities, making it difficult to initially diagnose this condition. Based on the results of this review, the most frequent symptoms in DCM include unspecified paresthesias, hand numbness, clumsiness or paresthesias, weakness and gait impairment. Neck and/or shoulder pain was present in 51% of patients with DCM, whereas a minority had back (19%) or lower extremity pain (10%). With respect to autonomic symptoms, bladder dysfunction was uncommon although more frequent than bowel or sexual impairment. The current scoping review provides a framework to create a diagnostic toolkit for specialists, primary care physicians, and allied health professionals.
Acknowledgments
This research aligns with the AO Spine RECODE-DCM top research priority ‘Diagnostic Criteria’ selected by people living and working with DCM. For further information on how this process was conducted, why this question was prioritized, and global updates on currently aligned research, please visit aospine.org/recode/diagnostic-criteria. This article, including the broader efforts to establish diagnostic criteria for DCM, is led by the RECODE-DCM Diagnostic Criteria Incubator Group. This was initially launched, with support from AO Spine through the AO Spine Knowledge Forum Spinal Cord Injury, a focused group of international Spinal Cord Injury experts. The oversight and support of the incubator has now transitioned to Myelopathy.org, a global charity focused on DCM.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Benjamin Davies https://orcid.org/0000-0003-0591-5069
Konstantinos Margetis https://orcid.org/0000-0002-3715-8093
Andrea Boraschi https://orcid.org/0000-0002-2908-5234
Oke Righteous Obadaseraye https://orcid.org/0000-0001-8018-6076
Michael G. Fehlings https://orcid.org/0000-0002-5722-6364
Ratko Yurac https://orcid.org/0000-0003-3603-6294
Elizabeth A. Roberts https://orcid.org/0000-0003-2738-4203
Tanzil Rujeedawa https://orcid.org/0000-0002-7089-1684
References
- 1.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: Epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976). 2015;40(12):E675-E693. doi: 10.1097/BRS.0000000000000913 [DOI] [PubMed] [Google Scholar]
- 2.Davies BM, Khan DZ, Barzangi K. et al. We choose to call it ‘degenerative cervical myelopathy’: Findings of AO spine RECODE-DCM, an international and multi-stakeholder partnership to agree a standard unifying term and definition for a disease. Global Spine J. 2022:219256822211117. doi: 10.1177/21925682221111780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waqar M, Wilcock J, Garner J, Davies B, Kotter M. Quantitative analysis of medical students’ and physicians’ knowledge of degenerative cervical myelopathy. BMJ Open. 2020;10(1):e028455. doi: 10.1136/bmjopen-2018-028455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tetreault L, Lange SF, Chotai S. et al. A systematic review of definitions for neurological complications and disease progression in patients treated surgically for degenerative cervical myelopathy. Spine (Phila Pa 1976). 2019;44(18):1318-1331. doi: 10.1097/BRS.0000000000003066 [DOI] [PubMed] [Google Scholar]
- 5.Hilton B, Tempest-Mitchell J, Davies B, Kotter M. Route to diagnosis of degenerative cervical myelopathy in a UK healthcare system: A retrospective cohort study. BMJ Open. 2019;9(5):e027000. doi: 10.1136/bmjopen-2018-027000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tetreault L, Kopjar B, Cote P, Arnold P, Fehlings MG. A clinical prediction rule for functional outcomes in patients undergoing surgery for degenerative cervical myelopathy: Analysis of an international prospective multicenter data set of 757 subjects. J Bone Joint Surg Am. 2015;97(24):2038-2046. doi: 10.2106/JBJS.O.00189 [DOI] [PubMed] [Google Scholar]
- 7.Pope DH, Mowforth OD, Davies BM, Kotter MRN. Diagnostic delays lead to greater disability in degenerative cervical myelopathy and represent a health inequality. Spine (Phila Pa 1976). 2020;45(6):368-377. doi: 10.1097/BRS.0000000000003305 [DOI] [PubMed] [Google Scholar]
- 8.Davies BM, Phillips R, Clarke D. et al. Establishing the socio-economic impact of degenerative cervical myelopathy is fundamental to improving outcomes [AO spine RECODE-DCM research priority number 8]. Global Spine J. 2022;12(1_suppl):122s-129s. doi: 10.1177/21925682211039835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurol. 2010;16(3):176-187. doi: 10.1097/NRL.0b013e3181da3a29 [DOI] [PubMed] [Google Scholar]
- 10.Tetreault L, Kalsi-Ryan S, Benjamin D, et al. Degenerative cervical myelopathy: A practical approach to diagnosis. Global Spine J. 2022:21925682211072847. doi: 10.1177/21925682211072847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tetreault L, Goldstein CL, Arnold P. et al. Degenerative cervical myelopathy: A spectrum of related disorders affecting the aging spine. Spine Neurosurgery 2015;77(Suppl 4):S51-S67. doi: 10.1227/NEU.0000000000000951 [DOI] [PubMed] [Google Scholar]
- 12.Hilton B, Gardner EL, Jiang Z. et al. Establishing diagnostic criteria for degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 3]. Global Spine J. 2022;12(1_suppl):55S-63S. doi: 10.1177/21925682211030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies BM, Khan DZ, Mowforth OD. et al. RE-CODE DCM (REsearch objectives and common data elements for degenerative cervical myelopathy): A consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for clinical research and the definition of the research priorities. Global Spine J. 2019;9(1 Suppl):65S-76S. doi: 10.1177/2192568219832855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tricco AC, Lillie E, Zarin W. et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467-473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 15.Davies BM, Goh S, Yi K, Kuhn I, Kotter MRN. Development and validation of a MEDLINE search filter/hedge for degenerative cervical myelopathy. BMC Med Res Methodol. 2018;18(1):73. doi: 10.1186/s12874-018-0529-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MA, Mowforth OD, Kuhn I, Kotter MRN, Davies BM. Development of a validated search filter for Ovid Embase for degenerative cervical myelopathy. Health Info Libr J. 2023;40:181-189. doi: 10.1111/hir.12373 [DOI] [PubMed] [Google Scholar]
- 17.Hori M, Fukunaga I, Masutani Y. et al. New diffusion metrics for spondylotic myelopathy at an early clinical stage. Eur Radiol. 2012;22(8):1797-1802. doi: 10.1007/s00330-012-2410-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung PWH, Wong CKH, Lau ST, Cheung JPY. Psychometric validation of the adapted traditional Chinese (Hong Kong) version of the Japanese orthopaedic association cervical myelopathy evaluation questionnaire (JOACMEQ). Spine (Phila Pa 1976). 2018;43(4):E242-E249. doi: 10.1097/BRS.0000000000002287 [DOI] [PubMed] [Google Scholar]
- 19.Cook C, Roman M, Stewart KM, Leithe LG, Isaacs R. Reliability and diagnostic accuracy of clinical special tests for myelopathy in patients seen for cervical dysfunction. J Orthop Sports Phys Ther. 2009;39(3):172-178. doi: 10.2519/jospt.2009.2938 [DOI] [PubMed] [Google Scholar]
- 20.Audat ZA, Fawareh MD, Radydeh AM, et al. Anterior versus posterior approach to treat cervical spondylotic myelopathy, clinical and radiological results with long period of follow-up. Sage Open Med. 2018;6:2050312118766199. doi: 10.1177/2050312118766199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed OEF, Galal A. Multiple level anterior cervical discectomy and fusion versus posterior laminectomy for the management of multilevel cervical spondylotic myelopathy: clinical and radiological outcome. Egypt J Neurol Psychiatr Neurosurg. 2020;56(32):32. [Google Scholar]
- 22.Asher AL, Devin CJ, Kerezoudis P. et al. Comparison of outcomes following anterior vs posterior fusion surgery for patients with degenerative cervical myelopathy: An analysis from quality outcomes database. Neurosurgery. 2019;84(4):919-926. doi: 10.1093/neuros/nyy144 [DOI] [PubMed] [Google Scholar]
- 23.Burkhardt BW, Brielmaier M, Schwerdtfeger K, Sharif S, Oertel JM. Smith-Robinson procedure with and without Caspar plating as a treatment for cervical spondylotic myelopathy: A 26-year follow-up of 23 patients. Eur Spine J. 2017;26(4):1246-1253. doi: 10.1007/s00586-017-4988-8 [DOI] [PubMed] [Google Scholar]
- 24.Chacko AG, Turel MK, Sarkar S, Prabhu K, Daniel RT. Clinical and radiological outcomes in 153 patients undergoing oblique corpectomy for cervical spondylotic myelopathy. Br J Neurosurg. 2014;28(1):49-55. doi: 10.3109/02688697.2013.815326 [DOI] [PubMed] [Google Scholar]
- 25.Chatley A, Kumar R, Jain VK, Behari S, Sahu RN. Effect of spinal cord signal intensity changes on clinical outcome after surgery for cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(5):562-567. doi: 10.3171/2009.6.SPINE091 [DOI] [PubMed] [Google Scholar]
- 26.Chibbaro S, Mirone G, Makiese O, George B. Multilevel oblique corpectomy without fusion in managing cervical myelopathy: Long-term outcome and stability evaluation in 268 patients. J Neurosurg Spine. 2009;10(5):458-465. doi: 10.3171/2009.1.SPINE08186 [DOI] [PubMed] [Google Scholar]
- 27.Cho CB, Chough CK, Oh JY, Park HK, Lee KJ, Rha HK. Axial neck pain after cervical laminoplasty. J Korean Neurosurg Soc. 2010;47(2):107-111. doi: 10.3340/jkns.2010.47.2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi YA, Kim K. Stenosis and neurologic level discrepancies in cervical spondylotic myelopathy. Pharm Manag PM R. 2018;10(10):1051-1055. doi: 10.1016/j.pmrj.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 29.Chiu AYY, Pang MYC. Assessment of psychometric properties of various balance assessment tools in persons with cervical spondylotic myelopathy. J Orthop Sports Phys Ther. 2017;47(9):673-682. doi: 10.2519/jospt.2017.7283 [DOI] [PubMed] [Google Scholar]
- 30.Cui JL, Li X, Chan TY, Mak KC, Luk KD, Hu Y. Quantitative assessment of column-specific degeneration in cervical spondylotic myelopathy based on diffusion tensor tractography. Eur Spine J. 2015;24(1):41-47. doi: 10.1007/s00586-014-3522-5 [DOI] [PubMed] [Google Scholar]
- 31.Cole TS, Almefty KK, Godzik J. et al. Functional improvement in hand strength and dexterity after surgical treatment of cervical spondylotic myelopathy: A prospective quantitative study. J Neurosurg Spine. 2020;32:907-913. doi: 10.3171/2019.10.SPINE19685 [DOI] [PubMed] [Google Scholar]
- 32.Dong F, Wu Y, Song P. et al. A preliminary study of 3.0-T magnetic resonance diffusion tensor imaging in cervical spondylotic myelopathy. Eur Spine J. 2018;27(8):1839-1845. doi: 10.1007/s00586-018-5579-z [DOI] [PubMed] [Google Scholar]
- 33.Du W, Wang L, Shen Y, Zhang Y, Ding W, Ren L. Long-term impacts of different posterior operations on curvature, neurological recovery and axial symptoms for multilevel cervical degenerative myelopathy. Eur Spine J. 2013;22(7):1594-1602. doi: 10.1007/s00586-013-2741-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Ghandour NMF, Soliman MAR, Ezzat AAM, Mohsen A, Zein-Elabedin M. The safety and efficacy of anterior versus posterior decompression surgery in degenerative cervical myelopathy: A prospective randomized trial. J Neurosurg Spine. 2020;33:288-296. doi: 10.3171/2020.2.SPINE191272 [DOI] [PubMed] [Google Scholar]
- 35.Gembruch O, Jabbarli R, Rashidi A. et al. Degenerative cervical myelopathy in higher-aged patients: How do they benefit from surgery? J Clin Med. 2019;9(1):62. doi: 10.3390/jcm9010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerling MC, Radcliff K, Isaacs R. et al. Two-year results of the prospective spine treatment outcomes study: An analysis of complication rates, predictors of their development, and effect on patient derived outcomes at 2 years for surgical management of cervical spondylotic myelopathy. World Neurosurg. 2017;106:247-253. doi: 10.1016/j.wneu.2017.06.147 [DOI] [PubMed] [Google Scholar]
- 37.He S, Hussain N, Zhao J, Fu Q, Hou T. Improvement of sexual function in male patients treated surgically for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2006;31(1):33-36. doi: 10.1097/01.brs.0000192726.53415.bf [DOI] [PubMed] [Google Scholar]
- 38.Holly LT, Ellingson BM, Salamon N. Metabolic imaging using proton magnetic spectroscopy as a predictor of outcome after surgery for cervical spondylotic myelopathy. Clin Spine Surg. 2017;30(5):E615-E619. doi: 10.1097/BSD.0000000000000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;10(3):194-200. doi: 10.3171/2008.12.SPINE08367 [DOI] [PubMed] [Google Scholar]
- 40.Hossam MSM, Shabaan M, Abd Elsame M. Median cervical corpectomy for cervical myelopathy associated with ossified posterior longitudinal ligament. Trends Med Res. 2013;8:1-15. [Google Scholar]
- 41.Hou X, Lu S, Wang B, Kong C, Hu H. Morphologic characteristics of the deep cervical paraspinal muscles in patients with single-level cervical spondylotic myelopathy. World Neurosurg. 2020;134:e166-e171. doi: 10.1016/j.wneu.2019.09.162 [DOI] [PubMed] [Google Scholar]
- 42.Jain SSS, Singh S, Joshi AK, Pamecha C, Dave B, Patel P. Comparative study of anterior versus posterior decompression in elderly patients of cervical myelopathy with co-morbid conditions. Eur J Orthop Surg Traumatol. 2009;19:397-401. [Google Scholar]
- 43.Kang X, Xiang S, Pei S, Li S, Wang Q. Limited laminectomy with lateral mass screw fixation versus normal laminetomy for multi-segment cervical spondylotic myelopathy: a comparative analysis. Int J Clin Exp Med. 2020;13(4):2295-2303. [Google Scholar]
- 44.Kato M, Nakamura H, Konishi S. et al. Effect of preserving paraspinal muscles on postoperative axial pain in the selective cervical laminoplasty. Spine (Phila Pa 1976). 2008;33(14):E455-E469. doi: 10.1097/BRS.0b013e318178e607 [DOI] [PubMed] [Google Scholar]
- 45.Kawakita E, Kasai Y, Uchida A. Low back pain and cervical spondylotic myelopathy. J Orthop Surg. 2009;17(2):187-189. doi: 10.1177/230949900901700213 [DOI] [PubMed] [Google Scholar]
- 46.Kim CR, Yoo JY, Lee SH, Lee DH, Rhim SC. Gait analysis for evaluating the relationship between increased signal intensity on t2-weighted magnetic resonance imaging and gait function in cervical spondylotic myelopathy. Arch Phys Med Rehabil. 2010;91(10):1587-1592. doi: 10.1016/j.apmr.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 47.Kim IS, Kim YI, Hong JT, Lee DS. Rationales for a Urodynamic Study in Patients with Cervical Spondylotic Myelopathy. World Neurosurg. 2018;91(10):1587-1592. doi: 10.1016/j.wneu.2018.12.049 [DOI] [PubMed] [Google Scholar]
- 48.Kim YJ, Oh SH, Yi HJ, Kim YS, Ko Y, Oh SJ. Myelopathy caused by soft cervical disc herniation: Surgical results and prognostic factors. J Korean Neurosurg Soc. 2007;42(6):441-445. doi: 10.3340/jkns.2007.42.6.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiris T, Kilincer C. Cervical spondylotic myelopathy treated by oblique corpectomy: A prospective study. Neurosurgery. 2008;62(3):674-682. doi: 10.1227/01.neu.0000317316.56235.a7 [DOI] [PubMed] [Google Scholar]
- 50.Kommu R, Sahu BP, Purohit AK. Surgical outcome in patients with cervical ossified posterior longitudinal ligament: A single institutional experience. Asian J Neurosurg. 2014;9(4):196-202. doi: 10.4103/1793-5482.146602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong W, Xin Z, Du Q, Cao G, Liao W. Anterior percutaneous full-endoscopic transcorporeal decompression of the spinal cord for single-segment cervical spondylotic myelopathy: The technical interpretation and 2 years of clinical follow-up. J Orthop Surg Res. 2019;14(1):461. doi: 10.1186/s13018-019-1474-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409. doi: 10.1016/j.jocn.2008.07.070 [DOI] [PubMed] [Google Scholar]
- 53.Lau D, Winkler EA, Than KD, Chou D, Mummaneni PV. Laminoplasty versus laminectomy with posterior spinal fusion for multilevel cervical spondylotic myelopathy: Influence of cervical alignment on outcomes. J Neurosurg Spine. 2017;27(5):508-517. doi: 10.3171/2017.4.SPINE16831. [DOI] [PubMed] [Google Scholar]
- 54.Lo YL. The role of electrophysiology in the diagnosis and management of cervical spondylotic myelopathy. Ann Acad Med Singap. 2007;36(11):886-893. [PubMed] [Google Scholar]
- 55.Misawa T, Kamimura M, Kinoshita T, Itoh H, Yuzawa Y, Kitahara J. Neurogenic bladder in patients with cervical compressive myelopathy. J Spinal Disord Tech. 2005;18(4):315-320. doi: 10.1097/01.bsd.0000166638.31398.14 [DOI] [PubMed] [Google Scholar]
- 56.Moussellard HP, Meyer A, Biot D, Khiami F, Sariali E. Early neurological recovery course after surgical treatment of cervical spondylotic myelopathy: A prospective study with 2-year follow-up using three different functional assessment tests. Eur Spine J. 2014;23(7):1508-1514. doi: 10.1007/s00586-014-3315-x [DOI] [PubMed] [Google Scholar]
- 57.Niu S, Anastasio AT, Maidman SD, Faraj RR, Rhee JM. The frequency of various “myelopathic symptoms” in cervical myelopathy: Evaluation in a large surgical cohort. Clin Spine Surg. 2020;33(10):E448-E453. doi: 10.1097/BSD.0000000000000968 [DOI] [PubMed] [Google Scholar]
- 58.Rajasekaran S, Aiyer SN, Shetty AP, Kanna RM, Maheswaran A, Shetty JY. Effectiveness of Riluzole as a pharmacotherapeutic treatment option for early cervical myelopathy: A double-blinded, placebo-controlled randomised controlled trial. Eur Spine J. 2016;25(6):1830-1835. doi: 10.1007/s00586-015-4323-1 [DOI] [PubMed] [Google Scholar]
- 59.Raslan F, Koehler S, Berg F. et al. Vertebral body replacement with PEEK-cages after anterior corpectomy in multilevel cervical spinal stenosis: A clinical and radiological evaluation. Arch Orthop Trauma Surg. 2014;134(5):611-618. doi: 10.1007/s00402-014-1972-1 [DOI] [PubMed] [Google Scholar]
- 60.Revanappa KK, Moorthy RK, Alexander M, Rajshekhar V. Recovery of sympathetic skin response after central corpectomy in patients with moderate and severe cervical spondylotic myelopathy. Br J Neurosurg. 2017;31(2):199-204. doi: 10.1080/02688697.2016.1206178 [DOI] [PubMed] [Google Scholar]
- 61.Scholler K, Siller S, Brem C, Lutz J, Zausinger S. Diffusion tensor imaging for surgical planning in patients with cervical spondylotic myelopathy. J Neurol Surg Cent Eur Neurosurg. 2020;81(1):1-9. doi: 10.1055/s-0039-1691822 [DOI] [PubMed] [Google Scholar]
- 62.Sinha S, Jagetia A. Bilateral open-door expansive laminoplasty using unilateral posterior midline approach with preservation of posterior supporting elements for management of cervical myelopathy and radiculomyelopathy--analysis of clinical and radiological outcome and surgical technique. Acta Neurochir (Wien). 2011;153(5):975-984. doi: 10.1007/s00701-010-0872-6 [DOI] [PubMed] [Google Scholar]
- 63.Sugawara T, Hirano Y, Higashiyama N, Mizoi K. Limaprost alfadex improves myelopathy symptoms in patients with cervical spinal canal stenosis. Spine (Phila Pa 1976). 2009;34(6):551-555. doi: 10.1097/BRS.0b013e31819a84ec [DOI] [PubMed] [Google Scholar]
- 64.Thakar S, Christopher S, Rajshekhar V. Quality of life assessment after central corpectomy for cervical spondylotic myelopathy: Comparative evaluation of the 36-item short form health survey and the world health organization quality of life-bref. J Neurosurg Spine. 2009;11(4):402-412. doi: 10.3171/2009.4.SPINE08749 [DOI] [PubMed] [Google Scholar]
- 65.Thakar S, Rajshekhar V. Evaluation of pain as a preference-based health status measure in patients with cervical spondylotic myelopathy undergoing central corpectomy. Acta Neurochir (Wien). 2012;154(2):335-340. doi: 10.1007/s00701-011-1229-5 [DOI] [PubMed] [Google Scholar]
- 66.Turel MK, Sarkar S, Prabhu K, Daniel RT, Jacob KS, Chacko AG. Reduction in range of cervical motion on serial long-term follow-up in patients undergoing oblique corpectomy for cervical spondylotic myelopathy. Eur Spine J. 2013;22(7):1509-1516. doi: 10.1007/s00586-013-2724-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams KE, Paul R, Dewan Y. Functional outcome of corpectomy in cervical spondylotic myelopathy. Indian J Orthop. 2009;43(2):205-209. doi: 10.4103/0019-5413.50855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vitzthum HE, Dalitz K. Analysis of five specific scores for cervical spondylogenic myelopathy. Eur Spine J. 2007;16(12):2096-2103. doi: 10.1007/s00586-007-0512-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L, Liu CY, Tian JW, Tian X, Dong SH, Zhao QH. Combined single-level subtotal corpectomy and decompression for cervical spondylotic myelopathy treatment. ANZ J Surg. 2012;82(5):342-347. doi: 10.1111/j.1445-2197.2011.05996.x [DOI] [PubMed] [Google Scholar]
- 70.Wei L, Cao P, Xu C. et al. The relationship between preoperative factors and the presence of intramedullary increased signal intensity on T2-weighted magnetic resonance imaging in patients with cervical spondylotic myelopathy. Clin Neurol Neurosurg. 2019;178:1-6. doi: 10.1016/j.clineuro.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 71.Zhang RJ, Shen CL, Zhang JX. et al. Clinical features and surgical outcomes of cervical spondylotic myelopathy in patients of different ages: A retrospective study. Spinal Cord. 2018;56(1):7-13. doi: 10.1038/sc.2017.91 [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Liu H, Yang H, Pi B. Relationship between sagittal balance and axial symptoms in patients with cervical spondylotic myelopathy treated with anterior cervical discectomy and fusion. J Invest Surg. 2020;33(5):404-411. doi: 10.1080/08941939.2018.1524948 [DOI] [PubMed] [Google Scholar]
- 73.Zhou F, Huang M, Wu L. et al. Altered perfusion of the sensorimotor cortex in patients with cervical spondylotic myelopathy: an arterial spin labeling study. J Pain Res. 2018;11:181-190. doi: 10.2147/JPR.S148076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou FQ, Tan YM, Wu L, Zhuang Y, He LC, Gong HH. Intrinsic functional plasticity of the sensory-motor network in patients with cervical spondylotic myelopathy. Sci Rep. 2015;5:9975. doi: 10.1038/srep09975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chacko AG, Joseph M, Turel MK, Prabhu K, Daniel RT, Jacob KS. Multilevel oblique corpectomy for cervical spondylotic myelopathy preserves segmental motion. Eur Spine J. 2012;21(7):1360-1367. doi: 10.1007/s00586-011-2137-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chacko AG, Daniel RT. Multilevel cervical oblique corpectomy in the treatment of ossified posterior longitudinal ligament in the presence of ossified anterior longitudinal ligament. Spine (Phila Pa 1976). 2007;32(20):E575-E580. doi: 10.1097/BRS.0b013e31814b84fe [DOI] [PubMed] [Google Scholar]
- 77.Zipser CM, Fehlings MG, Margetis K, AO Spine RECODE DCM Steering Committee and Members of the Diagnostic Criteria Working Group et al. Proposing a framework to understand the role of imaging in degenerative cervical myelopathy: enhancement of MRI protocols needed for accurate diagnosis and evaluation. Spine (Phila Pa 1976). 2022;47(17):1259-1262. doi: 10.1097/BRS.0000000000004389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalsi-Ryan S, Rienmueller AC, Riehm L. et al. Quantitative assessment of gait characteristics in degenerative cervical myelopathy: A prospective clinical study. J Clin Med. 2020;9(3):752. doi: 10.3390/jcm9030752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tetreault L, Garwood P, Gharooni AA. et al. Improving assessment of disease severity and strategies for monitoring progression in degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 4]. Global Spine J. 2022;12(1_suppl):64S-77S. doi: 10.1177/21925682211063854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuhtz-Buschbeck JP, Johnk K, Mader S, Stolze H, Mehdorn M. Analysis of gait in cervical myelopathy. Gait Posture. 1999;9(3):184-189. doi: 10.1016/s0966-6362(99)00015-6 [DOI] [PubMed] [Google Scholar]
- 81.Haddas R, Patel S, Arakal R, Boah A, Belanger T, Ju KL. Spine and lower extremity kinematics during gait in patients with cervical spondylotic myelopathy. Spine J. 2018;18(9):1645-1652. doi: 10.1016/j.spinee.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 82.Lee JH, Lee SH, Seo IS. The characteristics of gait disturbance and its relationship with posterior tibial somatosensory evoked potentials in patients with cervical myelopathy. Spine (Phila Pa 1976). 2011;36(8):E524-E530. doi: 10.1097/BRS.0b013e3181f412d9 [DOI] [PubMed] [Google Scholar]
- 83.Malone A, Meldrum D, Bolger C. Gait impairment in cervical spondylotic myelopathy: Comparison with age- and gender-matched healthy controls. Eur Spine J. 2012;21(12):2456-2466. doi: 10.1007/s00586-012-2433-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki E, Nakamura H, Konishi S, Yamano Y. Analysis of the spastic gait caused by cervical compression myelopathy. J Spinal Disord Tech. 2002;15(6):519-522. doi: 10.1097/00024720-200212000-00015 [DOI] [PubMed] [Google Scholar]
- 85.Lannon M, Kachur E. Degenerative cervical myelopathy: Clinical presentation, assessment, and natural history. J Clin Med. 2021;10(16):3626. doi: 10.3390/jcm10163626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reid MC, Eccleston C, Pillemer K. Management of chronic pain in older adults. BMJ. 2015;350:h532. doi: 10.1136/bmj.h532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goode AP, Freburger J, Carey T. Prevalence, practice patterns, and evidence for chronic neck pain. Arthritis Care Res. 2010;62(11):1594-1601. doi: 10.1002/acr.20270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bot SD, van der Waal JM, Terwee CB. et al. Incidence and prevalence of complaints of the neck and upper extremity in general practice. Ann Rheum Dis. 2005;64(1):118-123. doi: 10.1136/ard.2003.019349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith ZA, Barry AJ, Paliwal M, Hopkins BS, Cantrell D, Dhaher Y. Assessing hand dysfunction in cervical spondylotic myelopathy. PLoS One. 2019;14(10):e0223009. doi: 10.1371/journal.pone.0223009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ono K, Ebara S, Fuji T, Yonenobu K, Fujiwara K, Yamashita K. Myelopathy hand. New clinical signs of cervical cord damage. J Bone Joint Surg Br. 1987;69(2):215-219. doi: 10.1302/0301-620X.69B2.3818752 [DOI] [PubMed] [Google Scholar]
- 91.Singjam A, Charoentanyarak K, Saengsuwan J. Prevalence and predictive factors for bilateral carpal tunnel syndrome by electrodiagnosis: A retrospective study. PLoS One. 2021;16(12):e0260578. doi: 10.1371/journal.pone.0260578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feloney MP, Leslie SW. Bladder Sphincter Dyssynergia. Treasure Island. FL: StatPearls; 2022. [PubMed] [Google Scholar]
- 93.Baldwin K, Ginsberg P, Harkaway RC. Under-reporting of erectile dysfunction among men with unrelated urologic conditions. Int J Impot Res. 2003;15(2):87-89. doi: 10.1038/sj.ijir.3900948 [DOI] [PubMed] [Google Scholar]
- 94.Comarr AE. Sexual function among patients with spinal cord injury. Urol Int. 1970;25(2):134-168. doi: 10.1159/000279669 [DOI] [PubMed] [Google Scholar]