Abstract
Study Design
Delayed diagnosis of degenerative cervical myelopathy (DCM) is likely due to a combination of its subtle symptoms, incomplete neurological assessments by clinicians and a lack of public and professional awareness. Diagnostic criteria for DCM will likely facilitate earlier referral for definitive management.
Objectives
This systematic review aims to determine (i) the diagnostic accuracy of various clinical signs and (ii) the association between clinical signs and disease severity in DCM?
Methods
A search was performed to identify studies on adult patients that evaluated the diagnostic accuracy of a clinical sign used for diagnosing DCM. Studies were also included if they assessed the association between the presence of a clinical sign and disease severity. The QUADAS-2 tool was used to evaluate the risk of bias of individual studies.
Results
This review identified eleven studies that used a control group to evaluate the diagnostic accuracy of various signs. An additional 61 articles reported on the frequency of clinical signs in a cohort of DCM patients. The most sensitive clinical tests for diagnosing DCM were the Tromner and hyperreflexia, whereas the most specific tests were the Babinski, Tromner, clonus and inverted supinator sign. Five studies evaluated the association between the presence of various clinical signs and disease severity. There was no definite association between Hoffmann sign, Babinski sign or hyperreflexia and disease severity.
Conclusion
The presence of clinical signs suggesting spinal cord compression should encourage health care professionals to pursue further investigation, such as neuroimaging to either confirm or refute a diagnosis of DCM.
Keywords: myelopathy, cervical, degenerative disc disease
Introduction
Degenerative cervical myelopathy (DCM) is a progressive spine condition and the most common cause of spinal cord dysfunction worldwide.1,2 Patients with DCM can present with subtle, non-specific symptoms in their upper and lower extremities, making it difficult to initially diagnose this condition.3,4 Behrbalk et al (2013) determined that the average time to diagnosis of DCM was 2.2 ± 2.3 years and that patients attended an average of 5.2 ± 3.6 physician visits before obtaining a correct diagnosis. 5 The first line physician was a primary care practitioner in 69% of cases and an orthopedic surgeon in 21% of cases. 5 DCM was most commonly mistaken for carpal tunnel syndrome or cervical disc radiculopathy without neurological deficit. Similarly, others have reported that the time between symptom onset and surgical evaluation was 17.7 ± 16.0 months. 6 This delay in diagnosis and assessment by a spinal surgeon has detrimental consequences and can result in incomplete postoperative recovery, impaired quality of life and significant disability including inability to work.7,8 DCM is often managed surgically with response to treatment dependent on degree of preoperative functional impairment and duration of symptoms.9,10 As such, timely diagnosis and management is critical in order to optimize outcomes.
Patients with DCM complain of symptoms in their upper and lower extremities, including bilateral arm paresthesia, reduced manual dexterity, gait instability, and weakness. Other symptoms include neck pain or stiffness, Lhermitte’s phenomena and urinary or fecal urgency or incontinence. On examination, patients with DCM typically present with bilateral motor and/or sensory deficits of the upper and lower extremities without facial involvement, although clinical variations can occur. 11 Furthermore, patients with DCM exhibit a combination of upper and lower motor neuron signs (manifestation of the disease that is identified during an examination) as well as abnormalities in the sensation of pain, temperature, proprioception and vibration. Upper motor neuron signs include hyperreflexia below the level of the lesion, Hoffmann sign, upgoing plantar responses, lower limb spasticity, and corticospinal distribution motor deficits. 11 Lower motor neuron signs result from compression of the nerve roots as they exit the spinal canal and commonly include muscle atrophy especially in the hands, muscle fasciculations and weakness. However, patients do not always present with clear signs of DCM, but rather demonstrate nonspecific dissociated sensorimotor deficits and subtle gait disturbances. Furthermore, common comorbidities in patients of typical DCM age, such as carpal tunnel syndrome, radiculopathy and arthritis, may further impede the diagnosis. Therefore, clinical signs of cervical myelopathy (ie spinal cord involvement) are of greatest diagnostic value.
Misdiagnosis or delayed diagnosis of DCM is likely associated with its subtle, non-specific symptoms, incomplete neurological assessments by clinicians and a lack of public and professional awareness.12,13 Developing diagnostic criteria for DCM will likely improve diagnosis and was determined as a research priority as part of the AO Spine RECODE-DCM (Research Objectives and Common Data Elements for Degenerative Cervical Myelopathy) project.14-16 The first step in this process is to identify candidate variables for inclusion in diagnostic criteria. Signs that exhibit high sensitivity and specificity, and those that are correlated with disease severity are likely the most relevant.
A previous systematic review by Cook et al (2011) aimed to evaluate the diagnostic accuracy of various clinical tests by summarizing studies that reported on sensitivity, specificity, and other metrics. 17 This review used the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool to assess the external and internal validity of a diagnostic study and evaluate its risk of bias. Based on this scoring system, only a single study by Cook et al (2009) was rated as high quality. 18 This review concluded that the test with the highest sensitivity was the inverted supinator sign (61%), followed by the suprapatellar tendon reflex (56%) and the Hoffmann sign (44%). 17 Although the presence of clonus and the Babinski sign were not sensitive findings, they were the most specific tests (92% and 96%, respectively) for confirming a diagnosis of DCM. This review must be expanded on for the following reasons: (i) the term DCM has been introduced since its publication; (ii) several relevant studies have been conducted since 2011, including some that assess the diagnostic value of novel signs; and (iii) this review did not assess the relationship between the presence of clinical signs and disease severity.
The objective of this study was to conduct a systematic review of the literature to address the following key questions (KQ):
KQ1: What is the diagnostic accuracy (ie sensitivity, specificity, positive or negative predictive value, positive or negative likelihood ratio) of clinical signs in patients with DCM?
KQ2: What is the association between clinical signs and disease severity in patients with DCM?
Materials and Methods
The systematic review was formatted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist. Neither informed consent nor Institutional Review Board approval were required due to the nature of the study. The review was not registered in PROSPERO or any other similar site.
Eligibility Criteria
Table 1 outlines the eligibility criteria with respect to the population of interest, clinical signs, outcomes and study design.
Table 1.
Inclusion and Exclusion Criteria.
| Characteristic | Inclusion | Exclusion |
|---|---|---|
| Population | Patients with cervical myelopathy secondary to spondylosis, disc herniation, ossification of the posterior longitudinal ligament, congenital stenosis or subluxation Managed conservatively or surgically Age >18 years |
Patient with traumatic spinal cord injury, thoracic or lumbar myelopathy, spondylosis without myelopathy, tumor or infection Patients with asymptomatic spinal cord compression Patients with DCM at a specific level |
| Clinical signs | Hoffmann Inverted supinator Finger escape Suprapatellar quadriceps Babinski Pectoralis reflex Hyperreflexia (biceps, triceps, quadriceps, ankle) Clonus Gait abnormalities Romberg Other |
Patient reported symptoms Patient reported outcome measures (eg NDI, SF-36, VAS, subjective questionnaires) Clinician reported outcome measures (eg mJOA, nurick, walking test, grip dynamometer, GRASSP, GaitRite) Imaging characteristics |
| Outcome | KQ1: Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio KQ2: Disease severity as measured by standardized outcome instruments (eg mJOA, nurick, 30-m walking test, NDI, SF-36) |
KQ1: Reliability, responsiveness to change, internal consistency KQ2: Non-standardized outcome instruments (eg subjective rating of symptoms) or imaging findings |
| Study design | Case-control, cohort, cross-sectional, observational studies KQ1: Studies that report frequency of clinical signs in a cohort of DCM patients KQ1: Studies that include an acceptable control group for comparison (eg individuals with cervical radiculopathy or axial neck pain with no myelopathic symptoms) KQ2: Studies that statistically evaluate the association between the presence of a clinical sign and disease severity |
Commentaries or opinions Systematic or narrative reviews Animal or biomechanical studies Studies with <15 patients |
NDI, neck disability index; SF-36, short form-36; VAS, visual analog scale; mJOA, modified Japanese Orthopedic Association; GRASSP, graded redefined assessment of strength, sensation and prehension; KQ, key question; DCM, degenerative cervical myelopathy.
Population
This review targeted adult patients (>18 years) with cervical myelopathy secondary to spondylosis, disc herniation, ossification of the posterior longitudinal ligament (OPLL), congenital stenosis or subluxation. Eligible studies consisted of patients treated surgically or managed conservatively. Studies were excluded if they included patients with traumatic spinal cord injury, thoracic or lumbar myelopathy, asymptomatic spinal cord compression, tumor or infection or if they focused only on patients with DCM at a specific level.
Clinical Signs
Studies were included if they discussed a clinical sign used for diagnosing DCM. Clinical signs of interest included, but were not limited to, hyperreflexia, upgoing plantar responses, Hoffmann, clonus and gait abnormalities. Studies were excluded if they only assessed patient symptoms, patient- or clinician-reported outcome measures, or imaging characteristics. Table 2 summarizes definitions of clinical signs and highlights what constitutes a positive test.
Table 2.
Description of Clinical Tests.
| Clinical Test | Description | Positive Finding |
|---|---|---|
| Hoffmann | The examiner stabilizes the middle finger of the patient by holding it just proximal to the distal interphalangeal joint and then flicks it | Abduction of the thumb and flexion of the other fingers |
| Babinski | The examiner applies stimulation with the blunt end of a tendon hammer to the plantar surface of the foot, moving from laterally to medially from the heel to the first metatarsal | Flexion of the great toe and fanning of the other toes |
| Tromner | The examiner stabilizes the patient’s middle finger by holding it just proximal to the distal interphalangeal joint, in a flexed position. The examiner then taps or flicks the volar surface of the distal middle finger | Hyperreflexia of the index finger or thumb |
| Hyperreflexia at brachioradialis | The examiner positions the patient’s arm with the lateral side upwards and tests the reflex by striking the lower end of the radius, just proximal to the wrist | Brisk and exaggerated contraction of the brachioradialis, causing supination of the forearm |
| Hyperreflexia at biceps | The examiner slightly supinates the patient’s forearm and tests the reflex by striking the biceps tendon | Brisk and exaggerated contraction of the biceps, causing flexion of the elbow |
| Hyperreflexia at triceps | The examiner places the patient’s hand on the contralateral shoulder, keeping it in a flexed position and tests the reflex by striking the triceps tendon | Brisk and exaggerated contraction of the triceps, causing extension of the elbow |
| Suprapatellar reflex | The examiner takes the weight of 1 of the patient’s knee and strikes the suprapatellar tendon | Brisk and exaggerated contraction of the quadriceps tendon, causing extension of the knee |
| Hyperreflexia at patella | The examiner takes the weight of 1 of the patient’s knee and strikes the patellar tendon | Brisk and exaggerated contraction of the quadriceps tendon, causing extension of the knee |
| Hyperreflexia at achilles | The examiner dorsiflexes 1 of the patient’s foot and strikes the achilles tendon | Brisk and exaggerated contraction of the gastrocnemius, causing plantarflexion of the ankle |
| Hyperactive pectoralis reflex | The examiner strikes the patient’s pectoralis tendon in the deltopectoral groove | Adduction and internal rotation of the shoulder |
| Scapulohumeral reflex | The examiner strikes the spine of the scapula and acromion of the patient in a caudal direction | Elevation of the scapula or abduction of the humerus |
| Inverted supinator sign | The examiner positions the patient’s forearm in a slightly pronated position and strikes the forearm, just proximal to the radial styloid process. Also called the inverted radial reflex | Elbow extension and/or finger flexion |
| Cross adductor reflex | The examiner positions the patient’s legs in abduction and strikes the surface of the adductor tendons which is located on the medial surface of the thigh, proximal to the knee | Hyperreflexia of ipsilateral leg with reflex adduction of contralateral leg |
| Clonus | The examiner takes the weight of the patient’s ankle and quickly dorsiflexes it | Three or more involuntary and rhythmic muscle contractions |
| Absence of/Hypoactive deep tendon reflexes | The reflexes are tested as described above | Absent tendon reflex or reduced tendon reflex (despite the use of accentuation manoeuvres) |
| Hand withdrawal reflex | The examiner grasps the patient’s palm and strikes the dorsum of the patient’s hand with a tendon hammer | Abnormal flexor response |
| Gait deviation | The patient is asked to walk | Any abnormalities in gait such as spasticity, ataxia or changes in base |
| Abnormal great toe proprioception | The patient closes his or her eyes and the examiner asks whether the first toe is moved up or down | The patient is not able to tell whether the toe was moved up or down |
| Sensory impairment | The examiner tests 1 or more of the following modalities Light touch – the examiner assesses different areas of the skin using a cotton ball and confirms whether the patient is able to feel the touch and whether it is the same, reduced or increased compared to the sternum or the opposite limb Pain - a sharp object such as a pin is used to test the same areas of the patient’s skin Temperature – a cold or warm object is used to test the same areas of the patient’s skin Vibration - the examiner places a vibrating tuning fork on the patient’s most distal joints and asks whether the patient is able to feel it and when it stops. The examiner moves the tuning fork more proximally if the patient cannot feel the vibration at the most distal joints |
The patient has reduced sensation to touch, pain, temperature, vibration and/or proprioception |
| Motor impairment | The power of all myotomes is assessed | Reduced strength |
| Pyramidal signs/Upper motor neuron signs | Hyperreflexia or pathological reflexes, increased tone, motor weakness | NA |
| Myelopathic hand | The power of adduction and extension of the ulnar 2 or 3 fingers is tested by first asking the patient to perform active movements and then testing power against resistance. The examiner then tests the patient’s grip by asking him or her to rapidly grip and release with these fingers | Loss of power of adduction and extension of the ulnar 2 or 3 fingers and an inability to rapidly grip and release with these fingers |
| Wazir | The patients' right wrist is rested in extension, supination and a relaxed position over the examiner's left distal forearm. The examiner then taps at the extended wrist around the palmaris longus tendon. | Exaggerated flexion of fingers, thumb and wrist |
| Romberg | A patient stands with feet together and eyes closed | The patient sways or fall |
| Walking romberg | A patient walks 5 meters with their eyes open and then walks 5 meters with their eyes closed | The patient sways or fall |
| Wasting or atrophy | The examiner observes a patient’s muscles | Atrophy of the muscle |
| Spasticity | The examiner assesses the passive resistance of movement of each muscle | Velocity-dependent increased tone (ie hypertonia) |
| Finger escape sign | The examiner observes a patient’s hand with fingers extended and adducted | Involuntary abduction of the fifth finger due weakness of intrinsic hand muscles |
| Fasciculations | The examiner observes for involuntary movement of muscles at rest | |
| Ataxia | Poor muscle control that causes clumsy voluntary movements and impaired coordination | NA |
| Grip release | The examiner counts the number of grip and release cycles a patient does in 10 seconds | Less than 20 grip and release cycles in 10 seconds |
| Hyperthesia | The sensation of all dermatomes is assessed | Increased sensitivity to tactile stimulation |
| Spurling’s sign | The examiner extends and laterally flexes the patient’s neck and applies axial compression | Pain that radiates down the ipsilateral arm in the direction of the corresponding dermatome |
| Posterior column dysfunction | Vibration and proprioception is assessed in the upper and lower extremities | Reduced sensation to vibration and proprioception |
| Spinal tenderness | The examiner palpates the vertebral column | Tenderness to palpation |
| Orthostatic hypotension | Blood pressure is measured when the patient is supine and standing |
Outcome
For KQ1, studies were included if they summarized the sensitivity, specificity, positive or negative predictive value or positive or negative likelihood ratio of a clinical sign. In some cases, sensitivity was calculated from the frequency of a clinical sign in a DCM population. Table 3 provides definitions and equations for metrics of diagnostic accuracy. For KQ2, studies were included if they evaluated the association between the presence of a clinical sign and disease severity, as measured using an appropriate outcome instrument.
Table 3.
Definitions and Equations for Metrics of Diagnostic Accuracy.
| Metric of Diagnostic Accuracy | Definition | Pearls | Relevant Equations |
|---|---|---|---|
| Sensitivity | How often a test is correctly positive in individuals with a particular disease | Important for ruling out individuals who do not have the disease | |
| Specificity | How often a test is correctly negative in individuals who do not have a particular disease | Important for ruling in individuals who have the disease | |
| Positive predictive value | The percentage of patients with a positive test who actually have the disease | The higher the disease prevalence, the higher the PPV of the test for the disease | |
| Negative predictive value | The percentage of patients with a negative test who do not have the disease | The lower the disease prevalence, the higher the NPV of the test for that disease | |
| Positive likelihood ratio | The probability that a positive test would be expected in a patient with the disease, divided by the probability that a positive test would be expected in an individual without the disease | How much the probability of the disease is increased if the test result is positive | |
| Negative likelihood ratio | The probability that a negative test would be expected in a patient with the disease, divided by the probability that a negative test would be expected in an individual without the disease | How much the probability of the disease is decreased if the test result is negative |
TP, true positive; FN, false negative; FP, false positive; PPV, positive predictive value; NPV, negative predictive value; TN, true negative; LR+, positive likelihood ratio; LR-, negative likelihood ratio.
Study Design
For KQ1, this review targeted studies that (i) summarized the frequency of clinical signs in a cohort of DCM patients or (ii) compared the frequency of clinical signs between DCM patients and a control group. An example of an appropriate control group is a group of individuals with cervical radiculopathy or axial neck pain with no myelopathic symptoms. For KQ2, this review aimed to identify studies that statistically evaluated the association between a clinical sign and a measure of disease severity. Studies were excluded if they were commentaries, opinions, animal or biomechanical studies, review articles or surveys or if they consisted of less than 15 patients.
Search, Study Selection and Data Collection Process
Studies were identified using 2 electronic databases: MEDLINE and Cumulative Index to Nursing and Allied Health Literature. The last search was performed on June 29, 2021. A detailed search strategy was developed. The strategy was first developed in MEDLINE and then appropriately modified for the other database. Only studies involving humans and written in English were considered for inclusion.
All titles and abstracts were evaluated in a standardized manner by 2 independent reviewers. The abstracts were sorted based on predefined inclusion criteria. Full text investigation was completed for potentially relevant studies. Disagreement between reviewers was resolved through discussion. The search strategy is illustrated in Appendix 1.
The following data were collected from each included article: inclusion and exclusion criteria; demographics of both patients and controls; clinical signs and relevant definitions; results on diagnostic accuracy; and results on the association between clinical signs and DCM severity. For each clinical sign, true and false positives and negatives were extracted, allowing for the calculation of sensitivity, specificity, positive and negative predictive values and likelihood ratios. In some cases, our calculations for these metrics of diagnostic accuracy differed from those reported in the primary study. Data were not extracted on potential clinical signs that could not be distinguished from symptoms based on full text analysis (eg “sensory abnormalities,” “gait difficulties,” “upper or lower extremity weakness.”)
Risk of Bias in Individual Studies
The upgraded QUADAS-2 tool is a 11-question instrument used to evaluate the quality of diagnostic accuracy studies. 19 This tool was used in this review in order to assess the risk of bias of individual studies. To apply the QUADAS-2 tool, the evaluator must answer “yes,” “no” or unclear” to 11 questions within 4 domains. In this review, the studies were rated as having “low” risk of bias if less than 4 questions were answered “no” or” unclear,” “moderate” risk if 4 to 8 questions were answered “no” or “unclear” and “high” risk if more than 8 questions were answered “no” or “unclear.”
Data Analysis
Forest plots were created using RevMan. From each article, we extracted the number of patients who had the disease and tested positive (true positive), did not have the disease and tested positive (false positive), had the disease and tested negative (false negative), and did not have the disease and tested negative (true negative). From these values, sensitivity and specificity were computed and plotted. In some studies, we estimated each value using prevalence data in combination with reported sensitivity and specificity. In other studies, only true positives were reported. The 95% confidence intervals for sensitivity and specificity were automatically generated by RevMan using standard error.
Results
Study Selection
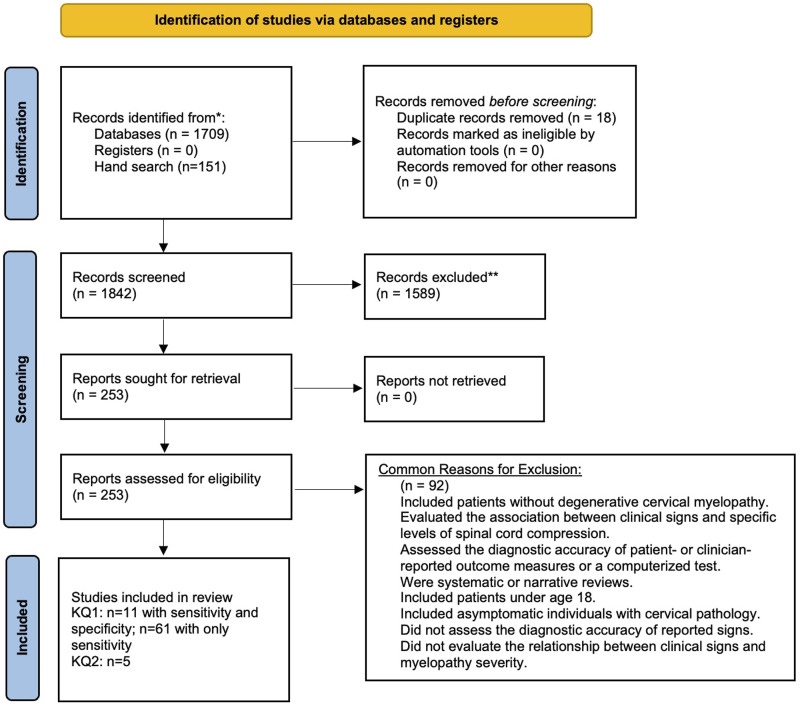
The search yielded a total of 1709 citations. An additional 151 studies were identified from a scoping review and by searching reference lists. Eighteen duplicate studies were removed. After abstract and title review, 1589 records were excluded. Two hundred and fifty-three studies were retrieved for full text investigation. Of these, eleven satisfied inclusion and exclusion criteria and had an appropriate control group. An additional 61 studies reported on frequency of clinical signs in a cohort of DCM patients and were also included. Commonly, studies were excluded if they (i) discussed cervical spine pathology in asymptomatic individuals; (ii) were systematic or narrative reviews; (iii) evaluated the association between clinical signs and specific levels of spinal cord compression; and (iv) assessed the diagnostic accuracy of patient- or clinician-reported outcome measures (Figure 1).
Figure 1.
An overview of the search process.
Study Characteristics
For KQ1, the search identified eleven studies that discussed the diagnostic accuracy of various clinical signs (Table 4, Figures 2, 3, 4).18,20-29 Sample sizes ranged from 45 to 7629. The most commonly reported clinical signs were Hoffmann (82%), Babinski (45%), hyperreflexia (36%) and inverted supinator sign (36%). All studies calculated sensitivity and specificity for at least 1 sign. Control groups included normal volunteers and patients with cervical spine complaints but no signs of myelopathy or evidence of cord compression. Of the included studies, 55% were prospective18,20-23,26 and 45% were retrospective.24,25,27-29 Sensitivity was calculated from 61 studies that reported on the frequency of clinical signs in a cohort of patients with DCM.10,30-89
Table 4.
Summary of Studies That Included a Control Group and Assessed the Diagnostic Accuracy of Various Clinical Signs.
| Author (Year), Study Design, QUADAS-2 (Risk of Bias)∞ | Objective | Cervical Myelopathy and Control Group | Demographic Information | Clinical Signs | Metrics of Diagnostic Accuracy Assessed |
|---|---|---|---|---|---|
| Rhee et al (2009), prospective, 4 (moderate) | To determine the prevalence and utility of commonly tested myelopathic signs in surgically treated patients with cervical myelopathy |
Cervical myelopathy group (n = 39) - History of myelopathic symptoms (upper extremity clumsiness, gait instability, nondermatomal numbness or weakness) - Presence of correlative spinal cord compression on advanced imaging - Treated with surgical decompression - Postoperative improvement in the modified nurick score of at least 1 point Control group (n = 37) - Neck pain or radicular arm complaints - No myelopathic symptoms - No evidence of cervical cord compression on advanced imaging - No history of previous spine surgery |
Cervical myelopathy group (n = 39) Age: 58 years Men: 56% Cord signal change: 51% Nurick preoperative 2.6 Control group (n = 37) Age: 48 Men: 49% Cord signal change: 0% Nurick preoperative: N/A |
Hyperreflexia (biceps, triceps, brachioradialis, patella, achilles) Hoffmann Inverted brachioradialis Sustained clonus Babinski |
Sensitivity Specificity Positive and negative predictive value a Positive and negative likelihood ratio a |
| Cook et al (2010), prospective, 5 (moderate) | To produce a cluster of predictive clinical findings for a sample of patients with cervical myelopathy |
Cervical myelopathy group (n = 88) - Diagnosis made by careful consideration of presenting symptoms, physical examination and imaging findings^ Control group (n = 161) - Cervical spine pain or dysfunction without image evidence of cervical myelopathy^ |
Cervical myelopathy group (n = 88) Age: 56.9±12.5 Men: 54.5% NDI, % disability: 40.3±19.4% SF-12: 42.9±7 Control group (n = 161) Age: 53.3±15.6 Men: 46.6% NDI, % disability: 40.4±18.5% SF-12: 45.4±6.7 |
Hoffmann Clonus Babinski Gait deviation Hyperreflexia (biceps, quadriceps, achilles) Inverted supinator sign |
Sensitivity Specificity Positive and negative predictive value a positive and negative likelihood ratio |
| Cook et al (2009), prospective, 2 (low) | To assess reliability and diagnostic accuracy of neurological tests associated with cervical myelopathy |
Cervical myelopathy group (n = 18) - Cervical spine pain with signal intensity changes on MRI confirming the presence of myelomalacia Control group (n = 27) - Cervical spine pain without MRI evidence of myelomalacia |
Age: 52±13.4 Men: 41% |
Hoffmann Hyperreflexia of biceps or triceps Inverted supinator sign Suprapatellar quadriceps Hand withdrawal reflex Babinski Clonus |
Sensitivity Specificity Positive and negative predictive value a positive and negative likelihood ratio |
| Chaiyamongkol et al (2017), prospective, 5 (moderate) | To investigate the significance of the tromner sign and how its presence correlates with the severity of cervical myelopathy |
Cervical myelopathy group (n = 36) - Clinical symptoms and physical signs of cervical myelopathy, including loss of hand dexterity, gait dysfunction, motor/sensory dysfunction and long tract signs - Evidence of corresponding cervical cord compression Control group (n = 14) - Normal volunteers and patients seeking orthopedic care for non-spine related issues |
Cervical myelopathy group (n = 36) Age: 59±9.9 Men: 77.8% Control group (n = 14) Age: 53.5±10.3 Men: 21.4% |
Hoffmann Tromner Inverted radial reflex Babinski |
Sensitivity Specificity a Positive and negative predictive value Positive and negative likelihood ratio a |
| Chang et al (2011), prospective, 3 (low) | To evaluate and quantify the tromner sign and compare its parameters to the severity of cord compression in patients with cervical myelopathy |
Cervical myelopathy group (n = 46) - Sensory impairment, muscular weakness or associated hyperreflexia in upper and lower extremities - MRI evidence of cord compression Control group (n = 30) - Age matched healthy spouses of patients |
Not reported | Tromner Hoffmann |
Sensitivity Specificity a Positive and negative predictive value a Positive and negative likelihood ratio a |
| Harrop et al (2010), retrospective, 5 (moderate) | To correlate clinical findings of myelopathic signs to cervical MRI features of cord compression and parenchymal changes on T2-weighted images |
Cervical myelopathy group (n = 54) - Presence of >1 long tract sign localized to the cervical spine, including hoffmann, babinski, clonus, hyperreflexia, crossed abductor and gait dysfunction Control group (n = 49) - Referred for symptomatic cervical spine disease but without myelopathic signs |
Cervical myelopathy group (n = 54) Age: 56.9 Men: 44% Control group (n = 49) Age: 49.5 Men: 47% |
Gait abnormality Hyperreflexia (LE or UE) LE hyperreflexia UE hyperreflexia Babinski Hoffmann Cross-abductor Sensory loss |
Sensitivity Specificity b Positive and negative predictive value b |
| Cao et al (2019), retrospective, 3 (low) | To analyze the correlation between the hoffmann sign and cervical pathology in symptomatic patients; to calculate the sensitivity, specificity, and positive and negative predictive values of the hoffmann sign for cervical pathology |
Cervical myelopathy group (n = 86)& - Referred for complaints related to the cervical spine - Image evidence of spinal cord compression Control group (n = 21) - Referred for complaints related to the cervical spine - No image evidence of spinal cord compression |
Not reported | Hoffmann | Sensitivity Specificity Positive and negative predictive value Positive and negative likelihood ratio a |
| Glaser et al (2001), prospective, 2 (low) | To evaluate the hoffmann sign as a screening tool for radiographic evidence of cervical spinal cord compression |
Cervical myelopathy group (n = 48)& - Referred for complaints related to the cervical spine - Image evidence of spinal cord compression Control group (n = 76) - Referred for complaints related to the cervical spine - No image evidence of spinal cord compression |
Not reported | Hoffmann | Sensitivity Specificity Positive and negative predictive value Positive and negative likelihood ratio a |
| Grijalva et al (2015), retrospective, 6 (moderate) | To examine the relationship between hoffmann sign and cervical pathology in symptomatic patients |
Cervical myelopathy group (n = 53)& - Neck pain or radicular arm complaints - Image evidence of spinal cord compression Control group (n = 118) - Neck pain or radicular arm complaints - No image evidence of spinal cord compression |
Not reported | Hoffmann | Sensitivity Specificity Positive and negative predictive value Positive and negative likelihood ratio a |
| Archer et al (2020), retrospective analysis of prospective data, 9 (high) | To develop and validate prediction models for patient-reported disability, pain and myelopathy outcomes at 12 months in patients undergoing cervical spine surgery |
Cervical myelopathy group (n = 2641) - No definition Control group (n = 4988) - Cervical radiculopathy |
Cervical myelopathy group (n = 2641) Age: 60.4±11.4 Men: 53% Current smoker: 21% Baseline mJOA 12.4±2.8 Control group (n = 4988) Age: 54.6±11.0 Men: 50% Current smoker: 20% |
Motor deficit, undefined | Sensitivity Specificity a Positive and negative predictive value a Positive and negative likelihood ratio a |
| Phillips 1975, retrospective, 6 (moderate) | To assess clinical differences between patients with cervical spondylosis with brachial neuritis and those with cervical myelopathy |
Cervical myelopathy group (n = 151) - Not defined Control group (n = 50) - Cervical spondylosis with brachial neuritis - No involvement of the lower limbs (ie no long tract compression in the spinal cord) - Symptoms and signs confined to the neck, shoulder girdle and upper limb - Predominant feature being pain in the upper limb or brachial neuralgia |
Cervical myelopathy group (n = 151) Age: 57 Duration of symptoms: 2.15 years Control group (n = 50) Age: 47.5 Duration of symptoms: 2.7 years |
Motor impairment Sensory impairment Absence of deep tendon reflexes Weakness and wasting of shoulder girdle and deltoid muscles |
Sensitivity Specificity a |
N/A, not applicable; NDI, neck disability index; SF-12, Short Form 12; MRI, magnetic resonance imaging; LE, lower extremity; UE, upper extremity; mJOA, modified Japanese Orthopedic.
∞QUADAS-2 is scored out of 11. Low risk of bias = zero to 3, moderate risk of bias = 4 to 8 and high risk of bias = 9 to 11.
^Anterior-posterior width reduction, cross-sectional evidence of cord compression, obliteration of the subarachnoid space and signal intensity changes to the cord were considered the most important imaging parameters for confirmation of DCM.
aNot reported in study but calculated using control group.
bonly reported for sensory loss. It was assumed that patients with a combination of cervical spine complaints and image evidence of spinal cord compression had cervical myelopathy
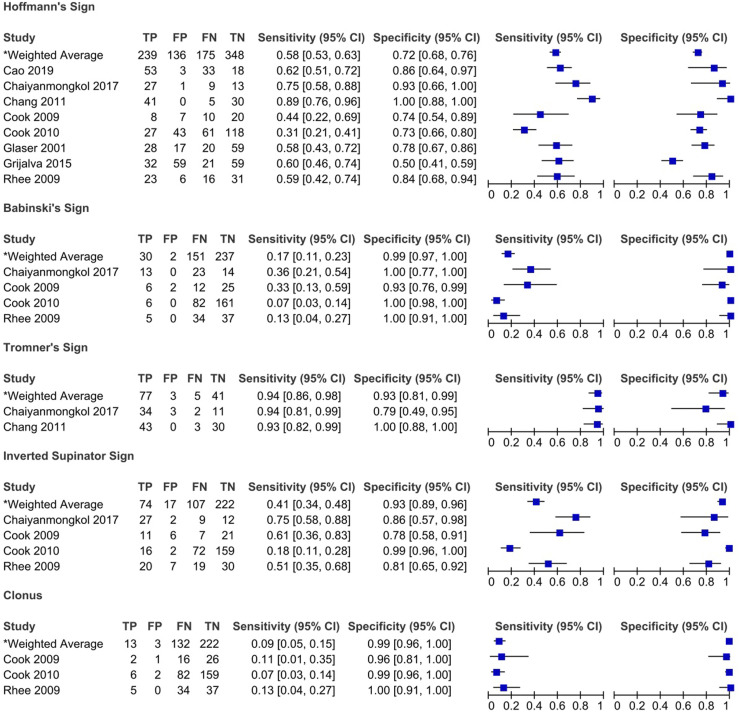
Figure 2.
Sensitivity and specificity of pathological reflexes in degenerative cervical myelopathy: Results of eleven studies that included a control group.
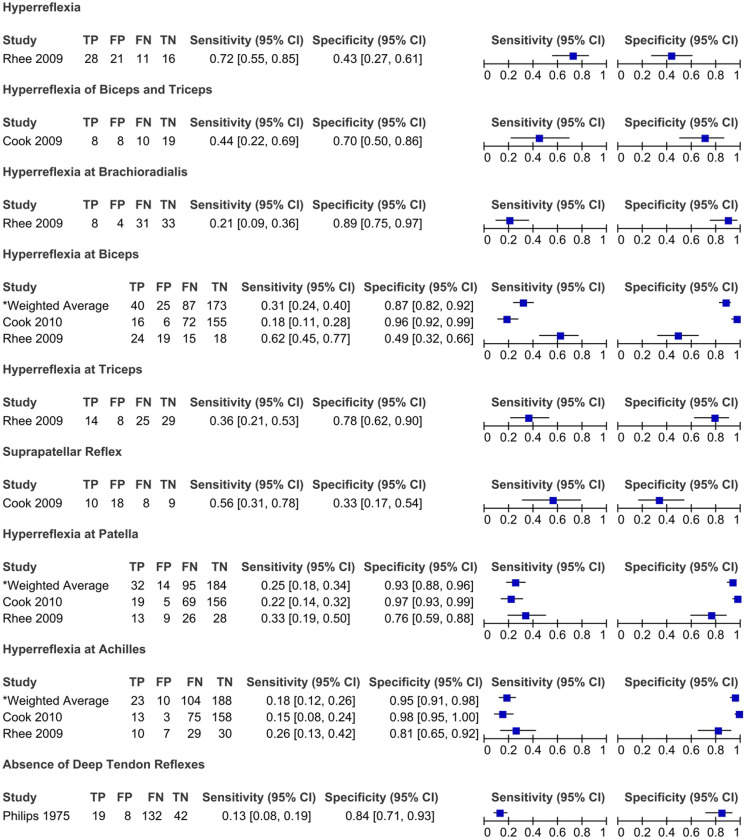
Figure 3.
Sensitivity and specificity of hyperreflexia and hyporeflexia in degenerative cervical myelopathy: Results of eleven studies that included a control group.
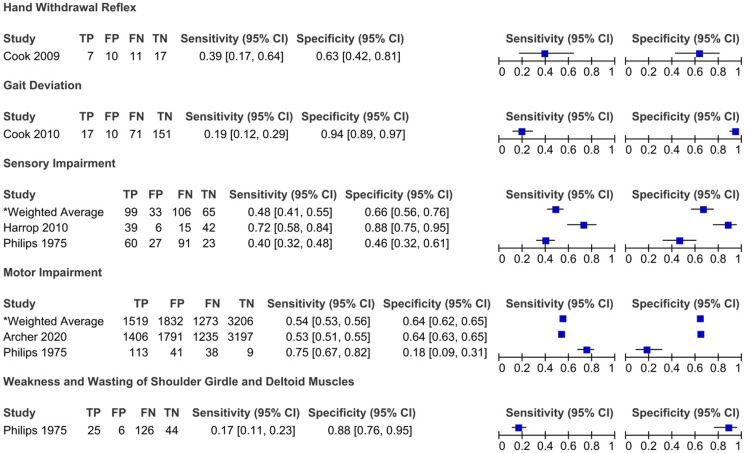
Figure 4.
Sensitivity and specificity of sensory and motor impairment and gait dysfunction in degenerative cervical myelopathy: Results of eleven studies that included a control group.
For KQ2, the search identified 5 studies that evaluated the association between various clinical signs and disease severity as measured by the JOA (n = 3),32,35,88 Nurick (n = 2),20,88 mJOA (n = 1), 36 European Myelopathy Score (EMS) (n = 1), 88 Prolo Score (n = 1) 88 and Cooper Myelopathy Scale (CMS) (n = 1). 88 The clinical signs that were discussed in these studies were Hoffmann (n = 4), Babinski (n = 4), hyperreflexia (n = 3), clonus (n = 2), inverted supinator sign (n = 1), dysdiadochokinesia (n = 1), pathological reflexes (n = 1), hypertonia (n = 1), paresis of the upper and lower extremity (n = 1) and great toe proprioception (n = 1).
Risk of Bias of Individual Studies
The QUADAS-2 scoring system was applied to the eleven studies that discussed diagnostic accuracy of various signs and included a control group. The risk of bias was deemed to be low in 4 studies,18,23,25,26 moderate in 620-22,24,27,29 and high in 1. 28
Results of Individual Studies
KQ1
What is the diagnostic accuracy (i.e. sensitivity, specificity, positive or negative predictive value, positive or negative likelihood ratio) of clinical signs in patients with DCM?
Studies that included a control group
Rhee et al (2009) compared the prevalence of various myelopathic signs between a cervical myelopathy group and a control group (patients with neck pain or radicular arm complaints with no image evidence of spinal cord compression or history of previous cervical surgeries). 20 Based on their results, patients with cervical myelopathy were more likely to exhibit any myelopathic (79% vs 57% in control group) or provocative sign (Hoffmann 59% vs 16%; inverted supinator sign 51% vs 19%; Babinski 13% vs 0%; and clonus 13% vs 0%) than individuals in the control group. In contrast, there were no significant differences in the frequency of hyperreflexia between the myelopathy and control groups. 20 Interestingly, 21% of patients diagnosed with cervical myelopathy, and ultimately treated surgically, demonstrated no myelopathic signs on examination. Biceps hyperreflexia had the highest sensitivity (62%), followed by Hoffmann sign (59%) and inverted brachioradialis (51%). In contrast, the sensitivities of Babinski (13%), clonus (13%) and hyperreflexia of the brachioradialis (21%) were low. The majority of myelopathic signs were specific, especially Babinski (100%) and clonus (100%).
Cook et al published 2 studies that evaluated the diagnostic accuracy of various clinical signs.18,21 In the 2009 study, patients were recruited if their primary complaint was cervical pain and were included in the cervical myelopathy group if they had signal intensity changes on MRI. 18 Based on their results, the inverted supinator sign had the highest sensitivity (61%), followed by the suprapatellar tendon reflex (56%), whereas Babinski (93%) and clonus (96%) demonstrated the highest specificity. 18 Based on likelihood ratios, only Babinski and inverted supinator sign exhibited significant diagnostic accuracy. Specifically, the positive likelihood ratio for Babinski was 4.5 with a post-test probability of 73%. This indicates that the probability of the disease increases by 73% if this test is positive. The negative likelihood ratio for the inverted supinator sign was .5 with a post-test probability of 25%. Cook et al (2009) also evaluated whether combining clinical signs affected diagnostic accuracy. 18 Unfortunately, the post-test probabilities of identifying DCM were not improved by combining clinical signs. In a second study, Cook et al (2010) aimed to develop a prediction model for the diagnosis of DCM. 21 Clinical signs that had a positive likelihood ratio greater than 2 or a negative likelihood ratio less than .5 were initially entered into a regression model and were retained if the P-value was less than .1. 21 The 4 clinical signs that were deemed the most important for the diagnosis of DCM were gait deviation, Hoffmann, inverted supinator sign and Babinski. Age greater than 45 was also included in the model. It was found with a sensitivity of 94% that a patient was unlikely to have DCM if they exhibited none or only a single 1 of these 5 variables. In contrast, the presence of any 3 of the 5 variables being positive yielded a specificity of 99% and a positive likelihood ratio of 30.9, with a post-test probability of 94%; implying a high probability of the patient having DCM. 21
Harrop et al (2010) evaluated the frequency of myelopathic signs in a cohort of patients with evidence of cervical spondylosis and symptoms of cervical stenosis. 24 Patients who exhibited greater than 1 long-tract sign localized to the cervical spinal cord comprised the myelopathy group, while those who did not were considered controls. 24 The sensitivity of signs ranged from 44% (Babinski) to 91% (gait abnormality), with hyperreflexia (85%), Hoffmann (83%) and lower extremity hyperreflexia (81%) demonstrating values above 80%. The presence of sensory loss was 72% sensitive and 88% specific for identifying cervical myelopathy and exhibited positive and negative predictive values of 87% and 74%, respectively.
Two studies evaluated the diagnostic accuracy of the Tromner sign.22,23 Based on their results, the sensitivity was 93% to 94% for identifying DCM and specificity ranged from 79% to 100%. The positive and negative predictive values were 92% to 100% and 85% to 91%, respectively. The study by Chang et al (2011) also assessed the sensitivity and specificity of the Tromner sign quantified by electrophysiological tests. 23 The sensitivity of this sign improved from 93% to 100% using this method. The diagnostic accuracy of the Hoffmann, inverted radial reflex and Babinski was also assessed in these 2 studies; results are summarized in Table 5.
Table 5.
Diagnostic Accuracy of Clinical Signs Used to Diagnose Degenerative Cervical Myelopathy: Results of Twelve Studies That Included a Control Group.
| Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Positive Likelihood Ratio | Negative Likelihood Ratio | |
|---|---|---|---|---|---|---|
| Hoffmann | ||||||
| Cao et al (2019) | 62 | 86 | 95 | 35 | 4.31 | .45 |
| Chaiyamongkol et al (2017) | 75 | 93 | 96 | 59 | 10.50 | .27 |
| Chang et al (2011) | 89 | 100 | 100 | 86 | Infinite | .11 |
| Cook et al (2009) | 44 | 74 | 53 | 67 | 1.71 | .75 |
| Cook et al (2010) | 31 | 73 | 39 | 66 | 1.15 | .95 |
| Glaser et al (2001) | 58 | 78 | 62 | 75 | 2.61 | .54 |
| Grijalva et al (2015) | 60 | 50 | 35 | 74 | 1.21 | .79 |
| Rhee et al (2009) | 59 | 84 | 79 | 66 | 3.64 | .49 |
| Babinski | ||||||
| Chaiyamongkol et al (2017) | 36 | 100 | 100 | 38 | Infinite | .64 |
| Cook et al (2009) | 33 | 93 | 75 | 68 | 4.50 | .72 |
| Cook et al (2010) | 7 | 100 | 100 | 66 | Infinite | .93 |
| Rhee et al (2009) | 13 | 100 | 100 | 52 | Infinite | .87 |
| Tromner | ||||||
| Chaiyamongkol et al (2017) | 94 | 79 | 92 | 85 | 4.41 | .071 |
| Chang et al (2011) | 93 | 100 | 100 | 91 | Infinite | .065 |
| Hyperreflexia | ||||||
| Rhee et al (2009) | 72 | 43 | 57 | 59 | 1.26 | .65 |
| Hyperreflexia of the biceps and triceps | ||||||
| Cook et al (2009) | 44 | 70 | 50 | 66 | 1.50 | .79 |
| Hyperreflexia at brachioradialis | ||||||
| Rhee et al (2009) | 21 | 89 | 67 | 52 | 1.90 | .89 |
| Hyperreflexia at biceps | ||||||
| Cook et al (2010) | 18 | 96 | 73 | 68 | 4.88 | .85 |
| Rhee et al (2009) | 62 | 49 | 56 | 54 | 1.20 | .79 |
| Hyperreflexia at triceps | ||||||
| Rhee et al (2009) | 36 | 78 | 64 | 54 | 1.66 | .82 |
| Hyperreflexia at patella | ||||||
| Cook et al (2010) | 22 | 97 | 79 | 69 | 6.95 | .81 |
| Rhee et al (2009) | 33 | 76 | 59 | 52 | 1.37 | .88 |
| Hyperreflexia at achilles | ||||||
| Cook et al (2010) | 15 | 98 | 81 | 68 | 7.93 | .87 |
| Rhee et al (2009) | 26 | 81 | 59 | 51 | 1.36 | .92 |
| Suprapatellar reflex | ||||||
| Cook et al (2009) | 56 | 33 | 36 | 53 | .83 | 1.33 |
| Inverted supinator sign | ||||||
| Chaiyamongkol et al (2017) | 75 | 86 | 93 | 57 | 5.25 | .29 |
| Cook et al (2009) | 61 | 78 | 65 | 75 | 2.75 | .50 |
| Cook et al (2010) | 18 | 99 | 89 | 69 | 14.64 | .83 |
| Rhee et al (2009) | 51 | 81 | 74 | 61 | 2.71 | .60 |
| Clonus | ||||||
| Cook et al (2009) | 11 | 96 | 67 | 62 | 3.00 | .92 |
| Cook et al (2010) | 7 | 99 | 75 | 66 | 5.49 | .94 |
| Rhee et al (2009) | 13 | 100 | 100 | 52 | Infinite | .87 |
| Absence of deep tendon reflexes | ||||||
| Philips (1975) | 13 | 84 | 70 | 24 | .79 | 1.04 |
| Hand withdrawal reflex | ||||||
| Cook et al (2009) | 39 | 63 | 41 | 61 | 1.05 | .97 |
| Gait deviation | ||||||
| Cook et al (2010) | 19 | 94 | 63 | 68 | 3.11 | .86 |
| Sensory impairment | ||||||
| Harrop et al (2010) | 72 | 88 | 87 | 74 | 5.78 | .32 |
| Philips (1975), of the upper limbs | 40 | 46 | 69 | 20 | .74 | 1.31 |
| Motor impairment | ||||||
| Archer et al (2020) | 53 | 64 | 44 | 72 | 1.48 | .73 |
| Philips (1975), of the upper limbs | 75 | 18 | 73 | 19 | .91 | 1.40 |
| Weakness and wasting of shoulder girdle and deltoid muscles | ||||||
| Philips (1975) | 17 | 88 | 81 | 26 | 1.38 | .95 |
Three studies evaluated the relationship between the presence of the Hoffmann sign and cervical pathology in symptomatic patients.25-27 In these studies, patients were considered to have cervical myelopathy if they were referred for complaints related to the cervical spine and had image evidence of cord compression. The control group consisted of individuals who were referred for similar symptoms but had no evidence of cord compression. Based on their results, the Hoffmann sign was 58% to 62% sensitive and 50% to 86% specific for identifying individuals with DCM.
Finally, 2 studies compared the rates of motor and sensory impairment between patient with cervical myelopathy and those with cervical radiculopathy or cervical spondylosis with brachial neuritis.28,29 The sensitivity of motor impairment ranged from 53% to 75% and specificity from 18% to 64%. Further results are provided in Table 5 for the diagnostic accuracy of sensory impairment, absence of deep tendon reflexes and weakness and wasting of shoulder girdle and deltoid muscles.
Studies that reported on frequency
Table 6 summarizes the results from the 61 studies that reported the frequency of clinical signs in a cohort of patients with DCM. Based on weighted averages, the most sensitive clinical signs for diagnosis DCM were hypertonia (.96, 95% CI 0.91 to .99) and pyramidal or upper motor neuron signs (.86, 95% CI 0.79 to .91) and hyperthesia (.81, 95% CI 0.70 to .89). The presence of hyperreflexia (.79, 95% CI 0.77 to .80) is also a sensitive sign that depends on location. Specifically, hyperreflexia of the lower extremities (.85, 95% CI 0.81 to .88) is more sensitive than hyperreflexia of the upper extremities (.78, 95% CI 0.74 to .82). The sensitivity of motor (.50, 95% CI 0.49 to .52) and sensory (.47, 95% CI 0.45 to .50) impairment increases when present in the upper extremities (motor: .73, 95% CI 0.69 to .77, sensory: .65, 95% CI 0.61 to .69) compared to the lower extremities (motor: .46, 95% CI 0.40 to .51, sensory: .62, 95% CI 0.52 to .72). Furthermore, while gait abnormalities have a sensitivity of only 47%, the presence of a spastic gait has a sensitivity of 69%. Based on single studies, the sensitivity of the following clinical signs was above 80%: myelopathic hand (.85, 95% CI 0.75 to .93) and patellar clonus (.80, 95% CI 0.61 to .92). Signs with poor sensitivity include motor deficit of individual muscle groups, clonus (.31, 95% CI 0.28 to .34), ataxia (.33, 95% CI 0.29 to .38), atrophy of intrinsic hand muscles (.38, 95% CI 0.35 to .41), Babinski (.42, 95% .41 to .44), and Romberg (.42, 95% .34 to .49).10,18,20-22,24,28-90 The supplemental material provides a complete summary of the average sensitivities of various clinical signs across included studies.
Table 6.
Sensitivity of Clinical Signs Used to Diagnose Degenerative Cervical Myelopathy.
| Clinical Sign | Sensitivity | Clinical Sign | Sensitivity |
|---|---|---|---|
| Hoffmann Acharya et al (2010) Ahmed and galal (2020) Alafifi et al (2007) Chatley et al (2009) Chikuda et al (2010) Chiu et al (2017) Du et al (2013) Du et al (2014) El-ghandour et al (2020) Findlay et al (2009) Funaba et al (2021) Harrop et al (2010) Holly et al (2009) Holly et al (2017) Hossam et al (2013) Hou et al (2020) Houten et al (2008) Kara et al (2011) Kong et al (2019) Lu et al (2017) Paholpak et al (2013) Tetreault et al (2015) Thakar et al (2009) Wang et al (2012) Watson et al (1997) Wei et al (2019) Wong et al (2004) |
86% 43% 45% 52% 81% 78% 44% 49% 25% 79% 76% 83% 43% 44% 97% 67% 68% (U: 21%, B: 47%) 56% 75% 69% 91% 63% 80% 42% 100% 63% 83% |
Babinski Acharya et al (2010) Ahmed and galal (2020) Alafifi et al (2007) Chikuda et al (2010) Chiles et al (1999) Chiu et al (2017) Di lazzaro et al (1992) Du et al (2013) Du et al (2014) El-ghandour et al (2020) Findlay et al (2009) Funaba et al (2021) Harrop et al (2010) Holly et al (2009) Holly et al (2017) Hossam et al (2013) Hou et al (2020) Houten et al (2008) Kara et al (2011) Kim et al (2010) Konya et al (2009) Lo (2007) Lu et al (2017) Paholpak et al (2013) Pilato et al (2021) Restuccia et al (1994) Tetreault et al (2015) Thakar et al (2009) Veilleux et al (1987) Wang et al (2012) Watson et al (1997) Wei et al (2019) Williams et al (2009) Zhang et al (2010) |
95% 53% 26% 53% 41% 44% 100% (U: 25%, B: 75%) 22% 30% 72% 31% 61% 44% 33% 38% 57% 33% 33% 63% 11% 55% 57% 52% 35% 61% 100% 36% 87% 46% 16% 93% 26% 79% 55% |
| Pathological reflexes Burkhardt et al (2017) Gerling et al (2017) Kong et al (2019) Vitzthum et al (2007) |
61% 17% 63% 65% |
Hypoactive reflexes Stetkarova et al (2009) Veilleux et al (1987) |
14% Biceps: 27%, triceps: 14% |
| Hyperreflexia Ahmed and galal (2020) Alafifi et al (2007) Burkhardt et al (2017) Chibbaro et al (2006) Chibbaro et al (2009) Chikuda et al (2010) Chiles et al (1999) Du et al (2013) Du et al (2014) El-ghandour et al (2020) Harrop et al (2010) Holly et al (2009) Holly et al (2017) Hossam et al (2013) Hou et al (2020) Houten et al (2008) Jain et al (2009) Kara et al (2011) Kim et al (2010) Konya et al (2009) Lo et al (2004) Lu et al (2017) Misra et al (1998) Raslan et al (2014) Stetkarova et al (2009) Tetreault et al (2015) Thakar et al (2009) Vitzthum et al (2007) Wei et al (2019) |
83% 82% 100% 66% 100% 94% 76% 66% 77% 83% 85% 62% 63% 97% 87% 60% 100% 100% 44% 55% 75% 71% 100% 33% 86% 78% 97% 79% 75% |
Clonus Acharya et al (2010) Ahmed and galal (2020) Alafifi et al (2007) Chikuda et al (2010) Chiles et al (1999) Chiu et al (2017) Du et al (2013) Du et al (2014) El-ghandour et al (2020) Holly et al (2009) Holly et al (2017) Hossam et al (2013) Kara et al (2011) Kim et al (2010) Paholpak et al (2013) Sinha and jagetia (2011) Thakar et al (2009) |
48% 27% 28% 35% 33% Ankle: 33% 17% 19% 60% 19% 19% Sustained patellar: 73%, unsustained patellar: 7%, sustained ankle: 73%, unsustained ankle: 3% 44% Ankle: 39% 31% UL: 65%, LL: 65% Ankle: 34% |
| Hyperreflexia of upper limbs Chiu et al (2017) Findlay et al (2009) Harrop et al (2010) Lo (2007) Lyu et al (2004) Sinha and jagetia (2011) Stetkarova et al (2009) Wang et al (2012) |
90% 72% 67% 85% 73% 100% 81% 60% |
Hyperreflexia of lower limbs Chiu et al (2017) Findlay et al (2009) Harrop et al (2010) Lo (2007) Lyu et al (2004) Restuccia et al (1994) Sinha and jagetia (2011) Stetkarova et al (2009) Veilleux et al (1987) |
76% 62% 81% 96% 96% 100% 100% 81% 81% |
| Hyperreflexia at biceps Acharya et al (2010) Imajo et al (2011) |
48% 70% |
Hyperreflexia at triceps Acharya et al (2010) Imajo et al (2011) |
5% 90% |
| Hyperreflexia at patella Acharya et al (2010) Funaba et al (2021) Wang et al (2012) |
95% 88% 69% |
Hyperreflexia at achilles Acharya et al (2010) Funaba et al (2021) |
90% 56% |
| Hyperactive pectoralis reflex Paholpak et al (2013) Watson et al (1997) |
32% 60% |
Inverted radial reflex Acharya et al (2010) Hossam et al (2013) Paholpak et al (2013) Wong et al (2004) |
90% 83% 89% 53% |
| Scapulohumeral reflex Paholpak et al (2013) |
20% | Cross adductor Harrop et al (2010) |
76% |
| Spasticity Alafifi et al (2007) Burkhardt et al (2017) El-ghandour et al (2020) Kara et al (2011) Kim et al (2007) Misra et al (1998) Restuccia et al (1994) Tetreault et al (2015) Wei et al (2019) |
LL: 20% 48% 51% UL: 25%, LL: 6% 58% 90% LL: 100% LL: 48% LL: 33% |
Romberg sign Chacko et al (2012) Findlay et al (2009) Kara et al (2011) Turel et al (2013) |
37% 34%; with walking: 74% 100% 43% |
| Hypertonia Chatley et al (2009) Jain et al (2009) Sinha and jagetia (2011) |
92% 100% UL: 100%, LL: 100% |
Gait abnormalities Audat et al (2018) Chacko et al (2014) Chibbaro et al (2006) Chibbaro et al (2009) Chiles et al (1999) Du et al (2014) Harrop et al (2010) Hossam et al (2013) Hou et al (2020) Lo (2004) Lo (2007) Lyu et al (2004) Raslan et al (2014) Tetreault et al (2015) Veilleux et al (1987) Wazir et al (2011) Wei et al (2019) |
41% Spastic: 95% Spastic: 41% Spastic: 66% Spastic: 68% 56% 91% 100% 53% 25% Spastic: 50% Spastic: 59% Ataxic: 67% Broad-based: 60% Ataxic: 33%, spastic: 63% 78% Broad-based: 44% |
| Ataxia Gembruch et al (2019) Scholler et al (2020) |
32% Spinal ataxia: 65% |
Pyramidal tetraparesis Moussellard et al (2014) |
28% |
| Pyramidal signs/Upper motor neuron signs Moussellard et al (2014) Stetkarova et al (2009) Wazir et al (2011) |
Pyramidal syndrome: 84% 48% 100% |
Motor deficit Audat et al (2018) Chatley et al (2009) Gembruch et al (2019) Gerling et al (2017) Hossam et al (2013) Jain et al (2009) Konya et al (2009) Lo et al (2004) Lu et al (2017) Tetreault et al (2015) Wei et al (2019) |
74% 84% 9% 22% 100% 57% 60% 75% 61% 55% 48% |
| Motor deficit, upper limbs Ahmed and galal (2020) Chiu et al (2017) Du et al (2014) Holly et al (2009) Holly et al (2017) Lo (2007) Lyu et al (2004) Sinha and jagetia (2011) Veilleux et al (1987) |
87% 90% 72% 52% 56% 54% 47% 100% 76% |
Motor deficit, lower limbs Ahmed and galal (2020) Chiu et al (2017) Du et al (2014) Holly et al (2009) Holly et al (2017) Lo (2007) Lyu et al (2004) Sinha and jagetia (2011) |
63% 47% 53% 29% 38% 26% 22% 91% |
| Motor deficit, deltoids Chiles et al (1999) Imajo et al (2011) |
11% 57% |
Motor deficit, biceps Chiles et al (1999) |
12% |
| Motor deficit, triceps Chiles et al (1999) |
29% | Motor deficit, hand intrinsics Chiles et al (1999) Thakar et al (2009) |
57% 69% |
| Motor deficit, iliopsoas Chiles et al (1999) |
39% | Motor deficit, quadriceps Chiles et al (1999) |
26% |
| Motor deficit, plantarflexion Chiles et al (1999) |
16% | Motor deficit, dorsiflexion Chiles et al (1999) |
18% |
| Atrophy of hand intrinsics Aggarwal et al (2016) Alafifi et al (2007) Chibbaro et al (2006) Chibbaro et al (2009) Misra et al (1998) Tetreault et al (2015) Thakar et al (2009) Wei et al (2019) |
41% 24% 37% 40% 35% 36% 80% 26% |
Muscular atrophy/Wasting Chatley et al (2009) Kim et al (2007) |
14% 27% |
| Fasciculations, lower limb Williams et al (2009) |
25% | Orthostatic hypotension Revannapa et al (2017) |
53% |
| Myelopathic hand sign Wazir et al (2011) |
85% | Wazir sign Wazir et al (2011) |
79% |
| Finger escape sign Findlay et al (2009) Paholpak et al (2013) Sugawara et al (2009) Wong et al (2004) |
48% 97% 29% 56% |
Grip and release Sugawara et al (2009) |
67% |
| Sensory impairment Ahmed and galal (2020) Audat et al (2018) Chatley et al (2009) Chibbaro et al (2009) El-ghandour et al (2020) Gembruch et al (2019) Gerling et al (2017) Holly et al (2009) Holly et al (2017) Hou et al (2020) Imajo (2011) Konya et al (2009) Lu et al (2017) Stetkarova et al (2009) Veilleux et al (1987) Wang et al (2012) Williams et al (2009) |
80% 88% 72% UL: 80% 51% 19% 25% UL: 38%, LL: 29% UL: 50%, LL: 38% Hands: 87% 100% 50% 75% 67% UL: 73%, LL: 86% 60% UL: 79%, LL: 71% |
Impaired proprioception and touch Chatley et al (2009) Di lazzaro et al (1992) Findlay et al (2009) Funaba et al (2021) Hou et al (2020) Lo (2007) Misra et al (1998) Moussellard et al (2014) Restuccia et al (1994) |
Proprioception: 31% 50% Proprioception at GT: 26% Proprioception at GT: 39% Proprioception: 47% Touch: 57% Proprioception at LL: 70% Proprioception: 76% Upper limbs: 45% |
| Impaired vibration Lyu et al (2004) Findlay et al (2009) |
Wrist: 47%, ankle: 65% Ankle: 60% |
Impaired pain and temperature Di lazzaro et al (1992) Kim et al (2007) Lo (2007) Lyu et al (2004) Misra et al (1998) Restuccia et al (1994) |
Upper limbs: 21% 27% Reduced pain: 52% Reduced pain: UL: 61%, LL: 65%, trunk a : 18% Reduced pain: 45% Upper limbs: 34% |
| Hyperthesia Du et al (2014) Hossam et al (2013) |
67% 100% |
Spinal tenderness Chatley et al (2009) |
20% |
| Spurling sign Hossam et al (2013) |
13% | Posterior column dysfunction Chacko et al (2014) Moussellard et al (2014) |
64% 42% |
U, unilateral; B, bilateral; UL, upper limb; LL, lower limb; GT, great toe.
aWith a level at the trunk.
KQ2
What is the association between clinical signs and disease severity in patients with DCM?
Five studies evaluated the association between the presence of various clinical signs and disease severity as measured by the Nurick, JOA or mJOA, EMS, CMS and Prolo scores (Table 7).20,32,35,36,88
Patients with a Hoffmann sign had more severe total JOA scores than those without this sign. 35 In single studies, a Hoffmann sign was also associated with worse upper and lower limb motor scores on the JOA.32,35 However, Funaba et al (2021) failed to detect a relationship between lower extremity JOA motor scores and the Hoffmann sign, 35 while Chikuda et al (2010) noted no correlation between a Hoffmann sign and upper extremity JOA motor scores. 32 Of note, patients with a unilateral Hoffmann sign were less severe than those with bilateral Hoffmann sign. 36 Compared to patients with a Babinski, those with a Hoffmann sign had higher (less severe) mJOA scores. 36 Finally, there were no associations between the Nurick score and the presence of a Hoffmann sign. 20
Two studies identified that patients with a Babinski had more severe lower extremity JOA motor scores than those without this clinical finding.32,35 In contrast, there was no association between a Babinski and total JOA, upper extremity JOA motor or Nurick scores.20,32,35 Single studies demonstrated that clonus was associated with more severe lower extremity JOA motor scores 32 but not with upper extremity JOA motor or Nurick scores.20,32 Furthermore, Vitzthum and Dalitz (2007) identified an association between (i) the presence of dysdiadochokinesia and worse JOA, CMS upper extremity and EMS scores; (ii) upper extremity weakness and worse JOA, CMS upper extremity and EMS scores; and (iii) lower extremity weakness and worse Nurick scores. Finally, this review determined no association between (i) greater toe proprioception and total or subscores of the JOA; 35 (ii) hyperreflexia and JOA, Nurick, CMS, EMS or Prolo scores; 32 and (iii) Nurick scores and hyperreflexia of the triceps, brachioradialis, patella and achilles or the inverted brachioradialis reflex. 20
Table 7.
The Association Between Various Clinical Signs and Disease Severity.
| Clinical Sign | Measurement Tool to assess disease severity | Statistical Methods | Main results | |
|---|---|---|---|---|
| Rhee et al (2009) | Hyperreflexia (biceps, triceps, brachioradialis, patella, achilles) Hoffmann Inverted brachioradialis Sustained clonus Babinski |
Preoperative nurick score | Wilcoxon rank sum test to assess difference in nurick score in patients with ≥1 myelopathic signs vs patients without myelopathic signs | - No statistically significant correlation between preoperative nurick score and the presence of any myelopathic sign - Mean preoperative nurick score in patients with ≥1 myelopathic sign was 2.6 vs 2.4 in patients without any signs (P = .4) |
| Houten et al (2008) | Hoffmann Unilateral hoffmann Bilateral hoffmann Babinski |
mJOA | Mann-whitney U-test to compare the mJOA scores in patients with a positive babinski sign and a positive hoffmann sign | - Mean mJOA score was higher in patients with a hoffmann sign (11.4) than patients with a babinski sign (9.6, P < .0001) - Mean mJOA score was higher in patients with unilateral hoffmann (12.8) than patients with bilateral hoffmann (10.8, P < .0001) |
| Chikuda et al (2010) | Hyperreflexia Hoffmann Babinski Clonus |
JOA (motor function of upper and lower extremity) | Mann-whitney U-test to analyze the relationship between pyramidal signs and upper and lower motor JOA scores | - No association between upper motor JOA scores and the presence of hyperreflexia, hoffmann, babinski and clonus - More severe lower motor JOA scores were associated with the presence of hoffmann (P = .031), babinski (P < .001) and clonus (P = .006) but not hyperreflexia (P = .445) |
| Funaba et al (2021) | Hoffmann Babinski Great toe proprioception |
Total, upper limb and lower limb JOA scores | Mann-whitney U-test to assess differences in JOA scores in patients with and without a particular clinical sign | - Hoffmann sign was associated with more severe total JOA (9.5 vs 10.4, P = .04) and upper limb JOA scores (2.92 vs 3.46, P = .032) - No association between hoffmann sign and lower limb JOA scores (2.79 vs 2.82, P = .92) - Babinski sign was associated with more severe lower limb JOA scores (2.64 vs 3.07, P = .046) - No association between babinski sign and total JOA (9.5 vs 10.1, P = .18) and upper limb JOA scores (3.05 vs 3.03, P = .85) - No association between great toe proprioception abnormalities and total JOA (P = .93), upper limb JOA (P = .62) or lower limb JOA scores (P = .62) |
| Vitzthum and dalitz (2007) | Dysdiadochokinesia Pathological reflexes Increased muscle tone Hyperreflexia of upper extremity Hyperreflexia of lower extremities Paresis of upper extremity Paresis of lower extremity |
Nurick, JOA, CMS, prolo, EMS | Mann-whitney U test to assess the association between clinical signs and various scores | - Presence of paresis of the lower extremity was associated with worse nurick scores (P < .05). No association between paresis of the lower extremity and JOA, CMS, prolo or EMS scores - Presence of paresis of the upper extremity was associated with worse JOA (P < .0001), CMS upper extremity (P < .0001) and EMS (P < .05) scores. No association between paresis of the upper extremity and nurick or prolo scores - Dysdiadochokinesia was associated with worse JOA (P < .05), CMS upper extremity (P < .0001) and EMS (P < .0001) scores. No association between dysdiadochokinesia and nurick or prolo scores - Pathological reflexes, increased muscle tone, and hyperreflexia of the upper and lower extremities were not associated with nurick, JOA, CMS, prolo or EMS scores |
mJOA, modified Japanese Orthopedic Association; JOA, Japanese Orthopedic Association; CMS, Cooper Myelopathy Scale; EMS, European Myelopathy Score.
Discussion
This systematic review aims to summarize the diagnostic accuracy of various clinical tests used to evaluate patients with DCM. Based on the results of the eleven studies, the most sensitive clinical tests were the Tromner sign and hyperreflexia, while the most specific tests were Babinski, the Tromner sign, clonus and the inverted supinator sign. Signs that may be eligible for inclusion in diagnostic criteria should demonstrate both moderate to high sensitivity and specificity. Of note, the majority of clinical signs identified in this review were either sensitive or specific, but not both. Furthermore, based on 1 series, examination findings may be absent entirely and a diagnosis of DCM can be made using a combination of patient-reported symptoms and imaging findings. As a result, it is important for clinicians to have a high index of suspicion for the diagnosis of DCM and to use a variety of clinical examination maneuvers to assess each patient. Furthermore, physicians should feel empowered to order imaging of the cervical spine (or even advanced imaging) if a patient demonstrates any of these signs of DCM or symptoms referable to the neck (please see separate study on symptoms of DCM). Given the impact of early surgery on neurological recovery and other patient outcomes, we must get to the point where subtle signs and symptoms of myelopathy trigger early referral for neuroimaging and assessment by experts. Timely diagnosis of DCM is a top priority to ensure these individuals either get the surgical treatment they need, or are at the very least, monitored for disease progression by physicians with significant expertise in DCM. The results from this systematic review and a scoping review on important DCM symptoms will be used in combination with expert opinion to generate diagnostic criteria that can guide a variety of health care professionals towards a diagnosis of DCM.
Upper Motor Neuron Signs
The diagnostic value of the Hoffmann sign has been extensively studied in individuals with cervical spine complaints. Based on the results of this review, the clinical utility of this sign is controversial given the wide range of sensitivities (25% to 100%), specificities (50% to 100%) and positive (1.15 to infinite) and negative (.11 to .95) likelihood ratios. Similar to other pathological reflexes, the Hoffmann sign results from decreased inhibitory input from the descending fibers of the corticospinal tract; this decreased inhibition causes exaggerated activation of motor neurons from connections with both sensory neurons and interneurons. 36 It is postulated that patients with peripheral neuropathy or radiculopathy may have a falsely negative Hoffmann sign due to interference with the normal reflex arc. 26 However, in a study by Houten and Noce (2008), the Hoffmann sign was evident in patients with known diabetic neuropathy despite normal to diminished deep tendon reflexes. 36 The technique of eliciting this reflex is challenging to master and interrater variability may contribute to the range of results across studies. Cook et al (2009), however, demonstrated substantial agreement (89%) between a neurosurgeon and a physical therapist in identifying a Hoffmann sign in a population of patients with cervical pain seeking a surgical consult. 18 Inter-rater reliability is likely to differ across specialists and may be lower when assessed by physicians who encounter these patients less frequently. Finally, a positive Hoffmann sign may be present in .7% to 3% of the population as well as in patients with upper motor neuron dysfunction from an intracranial process. 91 Although an asymmetric Hoffmann reflex may be more pathological, Houten and Noce (2008) demonstrated that a bilateral Hoffmann sign is associated with more definitive cord compression and more severe DCM. 36 This clinical sign should be used in combination with other signs and symptoms the support a diagnosis of myelopathy. Compared to the Hoffmann sign, the Tromner sign demonstrated superior sensitivity (94%) and specificity (93%) supporting its utility in both ruling in and out DCM.22,23
An upgoing plantar response, known as the Babinski sign, is a primitive reflex that disappears at 1 to 2 years of age but can reappear in the setting of upper motor neuron dysfunction. In the studies included in this review, the Babinski sign was highly specific for diagnosing cervical myelopathy. The control groups, however, were either healthy individuals or patients with cervical spine complaints without image evidence of spinal cord compression or myelomalacia. As such, the values for specificity may be falsely elevated as a Babinski sign may also be present in patients with noncervical or nonspondylotic disorders that result in upper motor neuron dysfunction. The reliability of the Babinski sign also ranges from poor to moderate in cohorts of individuals with and without a variety of neurological diseases.18,92,93 This variability in assessment stems from differences in technique as well as in interpretation. Of note, the Babinski sign requires both extension of the great toe and fanning of the other toes; withdrawal of the foot or isolated toe extension are not consistent with a positive test. Finally, patients with a Babinski sign had worse mJOA scores than those with a Hoffmann sign, indicating that release of this primitive reflex is associated with more severe corticospinal tract dysfunction. 36 The presence of clonus is also highly specific (99%) but demonstrates poor sensitivity.
Hyperreflexia at any deep tendon has moderate sensitivity for detecting cervical myelopathy (79%, with 47% of studies reporting a sensitivity over 80%). The sensitivity of this sign improves when assessing reflexes exclusively in the lower extremities (85%) compared with the upper extremities (78%).24,34,50 Evaluation of individual reflexes demonstrated a wide range of sensitivities: brachioradialis (21%), biceps (18% to 70%), triceps (5% to 90%), patella (22% to 95%) and achilles (15% to 90%). These results indicate that hyperreflexia, particularly in the lower extremities, may be an important screening tool for DCM. This finding is consistent with the underlying pathology of DCM as reflexes become exaggerated below the level of spinal cord compression due to reduced descending inhibition from the corticospinal tract. Furthermore, the reflexes in the upper extremities may be increased or decreased depending on the level of canal stenosis and the involvement of the nerve root as it exits the neural foramen. Presence of exaggerated reflexes in the upper extremities can further help to localize the level of spinal cord compression. For example, a hyperactive pectoralis reflex may indicate a myelopathic level above C4 whereas normal upper extremity reflexes are typically seen with a level at C6 to C7 or below.39,94 Notably, peripheral neuropathy may affect deep tendon reflexes; specifically, there was a significant difference in the frequency of achilles hyperreflexia in patients with diabetes (0%) compared to those without (35%, P = .04). 20 Caution should be taken in ruling out DCM in patients with peripheral neuropathy when reflexes are normal or diminished. Finally, the inverted supinator reflex has shown promising results with respect to specificity (93%) and therefore may be used to confirm a diagnosis of DCM in patients with cervical complaints.18,20-22,30
Sensorimotor and Gait Dysfunction
Patients with DCM will often have motor and sensory dysfunction of their upper and lower extremities. The sensitivity, however, is variable for motor deficits (9 to 100%) and low for impaired pain, proprioception, temperature and vibration sensation. Weakness in patients with DCM often begins in the intrinsic hand muscles and is likely due to anterior horn cell damage as opposed to nerve root compression. 33 As a result, hand weakness is more sensitive (60%) than motor deficits in the iliopsoas (39%), triceps (29%), quadriceps (26%), dorsiflexors (18%), plantarflexors (16%), biceps (12%) and deltoids (18%).33,70,89
Gait impairment may be 1 of the earliest manifestations of DCM. However, subtle changes in a patient’s stance and stability when walking may be difficult to appreciate early in the disease course. A myelopathic gait is often described as unsteady, broad-based or spastic and is a result of impaired proprioception in addition to upper motor neuron dysfunction. Furthermore, patients with DCM exhibit reduced gait velocity, a shortened stride length, increased double support time, a wide step width and slower cadence.95-99 DCM should be considered in patients with recurrent falls and gait deterioration. However, this clinical sign did not demonstrate high sensitivity given the wide variety of conditions that can impair gait, including osteoarthritis, peripheral neuropathy, obesity and vascular disease. Romberg sign can also be used to detect proprioception dysfunction but demonstrates poor sensitivity in DCM.30,34,37,51,84 When walking, however, the sensitivity of the Romberg sign increases from 34% to 74%. 34 This finding is clinically relevant as patients with DCM may sustain recurrent falls, especially in the dark. Overall, advanced gait assessments are essential to quantify subtle gait disturbances.
Based on the results of this systematic review, a combination of clinical signs is likely required to support a diagnosis of DCM. According to Cook et al (2010), the presence of 1 of the following has a high sensitivity (94%) for identifying individuals with DCM: gait deviation, Hoffmann sign, inverted supinator sign, Babinski sign and age over 45. 21 Given these results, DCM could be safely excluded in patients who did not have any of these 5 criteria. 21 Similarly, Rhee et al (2009) demonstrated that the presence of greater than or equal to 1 provocative sign (Hoffmann, inverted supinator sign, Babinski and clonus) was approximately 70% sensitive for diagnosing DCM. 20 However this study also identified cases of DCM with no positive examination findings. Furthermore, while the presence of an increasing number of clinical signs increases specificity and helps to confirm the diagnosis of DCM, it also appears to correlate to disease severity. Consequently, examination findings alone are not sufficient to make or exclude a diagnosis of DCM, especially early in the disease course.
Limitations
While this is the first review to summarize the sensitivity and specificity of clinical signs in DCM, there are limitations that should be mentioned. First, there were only eleven studies that included a control group in their analysis; as a result, there is limited information on the specificity of various DCM signs. Furthermore, of these eleven studies, 7 were rated as either moderate or high risk of bias on the QUADAS-2, questioning the validity of these results. In some of the studies, individuals were considered controls if they had neck, radicular or cervical spine pain or other symptoms but did not have myelomalacia or spinal cord compression on neuroimaging. It is increasingly recognized that patients can still be diagnosed with DCM in the absence of signal change or even spinal cord compression on static MRI. Moreover, several patients will have a combination of symptoms and signs of myelopathy and radiculopathy given that degenerative changes can simultaneous compress the spinal cord and nerve root. As such, these control groups may be suboptimal for assessing the accuracy of various symptoms. Other studies used patients referred to the orthopedic clinic for other complaints or healthy controls such as the spouses of patients. These reflect better control groups for the question of interest. Second, values for sensitivity and specificity are extracted from studies that are screening a particular population and not just a random group of individuals. Nonetheless, this review provides invaluable information on some of the most common signs of DCM and will undoubtedly improve understanding of this condition.
Conclusion and Future Directions
Patients with DCM can present with subtle, non-specific signs in their upper and lower extremities, making it difficult to initially diagnose this condition. Based on the results, the most sensitive clinical tests for diagnosing DCM were the Tromner sign and hyperreflexia, while the most specific tests were the Babinski, Tromner sign, clonus and inverted supinator sign. Signs that may be eligible for inclusion in diagnostic criteria should demonstrate both moderate to high sensitivity and specificity. While the presence of these clinical signs is helpful, examination findings may be absent entirely and a diagnosis of DCM can still be made using a combination of patient-reported symptoms and imaging findings. Furthermore, the absence of upper motor neuron signs does not rule out a diagnosis of DCM. In patients with clear symptoms of myelopathy, standard or advanced imaging, formal gait assessment and neurophysiology may be required to confirm the diagnosis. The current systematic review provides a framework to create a diagnostic toolkit for specialists, primary care physicians, and allied health professionals.
• Patients with DCM can present with subtle, non-specific signs in their upper and lower extremities, making it difficult to initially diagnose this condition.
• The most sensitive clinical tests for diagnosing DCM are the Tromner sign and hyperreflexia
• The most specific tests are the Babinski, Tromner sign, clonus and inverted supinator sign.
• Examination findings may be absent entirely and a diagnosis of DCM can still be made using a combination of patient-reported symptoms and imaging findings.
• Absence of upper motor neuron signs does not rule out a diagnosis of DCM.
Supplemental Material
Supplemental Material for The Value of Clinical Signs in the Diagnosis of Degenerative Cervical Myelopathy: Results of a Systematic Review by Zhilin Jiang, Benjamin Davies, Carl Zipser, Konstantinos Margetis, Allan Martin, Stavros Matsoukas, Freschta Zipser-Mohammadzada, Najmeh Kheram, Andrea Boraschi, Elina Zakin, Oke Righteous Obadaseraye, Michael G. Fehlings, Jamie Wilson, Ratko Yurac, Chad E Cook, Jamie Milligan, Julia Tabrah, Shirley Widdop, Lianne Wood, Elizabeth A. Roberts, Tanzil Rujeedawa, Lindsay Tetreault on behalf of the AO Spine RECODE-DCM Diagnostic Criteria Incubator in Global Spine Journal
Acknowledgments
This research aligns with the AO Spine RECODE-DCM top research priority ‘Diagnostic Criteria’ selected by people living and working with DCM. For further information on how this process was conducted, why this question was prioritized, and global updates on currently aligned research, please visit aospine.org/recode/diagnostic-criteria. This article, including the broader efforts to establish diagnostic criteria for DCM, is led by the RECODE-DCM Diagnostic Criteria Incubator Group. This was initially launched, with support from AO Spine through the AO Spine Knowledge Forum Spinal Cord Injury, a focused group of international Spinal Cord Injury experts. The oversight and support of the incubator has now transitioned to Myelopathy.org, a global charity focused on DCM.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Benjamin Davies https://orcid.org/0000-0003-0591-5069
Konstantinos Margetis https://orcid.org/0000-0002-3715-8093
Andrea Boraschi https://orcid.org/0000-0002-2908-5234
Oke Righteous ObadaserayeOrtho(UK) FMCOrtho https://orcid.org/0000-0001-8018-6076
Michael G. Fehlings https://orcid.org/0000-0002-5722-6364
Ratko Yurac https://orcid.org/0000-0003-3603-6294
Elizabeth A. Roberts https://orcid.org/0000-0003-2738-4203
Tanzil Rujeedawa https://orcid.org/0000-0002-7089-1684
References
- 1.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: Epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976). 2015;40(12):E675-E693. doi: 10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 2.Badhiwala JH, Ahuja CS, Akbar MA, et al. Degenerative cervical myelopathy - update and future directions. Nat Rev Neurol. 2020;16(2):108-124. doi: 10.1038/s41582-019-0303-0. [DOI] [PubMed] [Google Scholar]
- 3.Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. BMJ. 2018;360:k186. doi: 10.1136/bmj.k186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tetreault L, Kalsi-Ryan S, Benjamin D, et al. Degenerative cervical myelopathy: a practical approach to diagnosis. Global Spine J. 2022:21925682211072847. 10.1177/21925682211072847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrbalk E, Salame K, Regev GJ, Keynan O, Boszczyk B, Lidar Z. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg Focus. 2013;35(1):E1. doi: 10.3171/2013.3.FOCUS1374. [DOI] [PubMed] [Google Scholar]
- 6.Hilton B, Tempest-Mitchell J, Davies B, Kotter M. Route to diagnosis of degenerative cervical myelopathy in a UK healthcare system: a retrospective cohort study. BMJ Open. 2019;9(5):e027000. doi: 10.1136/bmjopen-2018-027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope DH, Mowforth OD, Davies BM, Kotter MRN. Diagnostic delays lead to greater disability in degenerative cervical myelopathy and represent a health inequality. Spine (Phila Pa 1976). 2020;45(6):368-377. doi: 10.1097/BRS.0000000000003305. [DOI] [PubMed] [Google Scholar]
- 8.Davies BM, Phillips R, Clarke D, et al. Establishing the socio-economic impact of degenerative cervical myelopathy is fundamental to improving outcomes [AO spine RECODE-DCM research priority number 8]. Global Spine J. 2022;12(1_suppl):122S-129S. doi: 10.1177/21925682211039835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tetreault L, Wilson JR, Kotter MRN, et al. Is preoperative duration of symptoms a significant predictor of functional outcomes in patients undergoing surgery for the treatment of degenerative cervical myelopathy? Neurosurgery. 2019;85(5):642-647. doi: 10.1093/neuros/nyy474. [DOI] [PubMed] [Google Scholar]
- 10.Tetreault L, Kopjar B, Cote P, Arnold P, Fehlings MG. A clinical prediction rule for functional outcomes in patients undergoing surgery for degenerative cervical myelopathy: Analysis of an international prospective multicenter data set of 757 subjects. J Bone Joint Surg Am. 2015;97(24):2038-2046. doi: 10.2106/JBJS.O.00189. [DOI] [PubMed] [Google Scholar]
- 11.Hilton B, Gardner EL, Jiang Z, et al. Establishing diagnostic criteria for degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 3]. Global Spine J. 2022;12(1_suppl):55S-63S. doi: 10.1177/21925682211030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tetreault L, Lange SF, Chotai S, et al. A systematic review of definitions for neurological complications and disease progression in patients treated surgically for degenerative cervical myelopathy. Spine (Phila Pa 1976). 2019;44(18):1318-1331. doi: 10.1097/BRS.0000000000003066. [DOI] [PubMed] [Google Scholar]
- 13.Davies BM, Mowforth O, Wood H, et al. Improving awareness could transform outcomes in degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 1]. Global Spine J. 2022;12(1_suppl):28S-38S. doi: 10.1177/21925682211050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies BM, Kwon BK, Fehlings MG, Kotter MRN. AO spine RECODE-DCM: Why prioritize research in degenerative cervical myelopathy? Global Spine J. 2022;12(1_suppl):5S-7S. doi: 10.1177/21925682211035379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tetreault L, Mowforth O, Khan DZ, et al. James lind alliance priority setting partnership for degenerative cervical myelopathy [AO spine RECODE-DCM]: An overview of the methodology used to process and short-list research uncertainties. Global Spine J. 2022;12(1_suppl):19S-27S. doi: 10.1177/21925682211062501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies BM, Khan DZ, Mowforth OD, et al. RE-CODE DCM (REsearch objectives and common data elements for degenerative cervical myelopathy): A consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for clinical research and the definition of the research priorities. Global Spine J. 2019;9(1 Suppl):65S-76S. doi: 10.1177/2192568219832855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook CE, Wilhelm M, Cook AE, Petrosino C, Isaacs R. Clinical tests for screening and diagnosis of cervical spine myelopathy: A systematic review. J Manip Physiol Ther. 2011;34(8):539-546. doi: 10.1016/j.jmpt.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Cook C, Roman M, Stewart KM, Leithe LG, Isaacs R. Reliability and diagnostic accuracy of clinical special tests for myelopathy in patients seen for cervical dysfunction. J Orthop Sports Phys Ther. 2009;39(3):172-178. doi: 10.2519/jospt.2009.2938. [DOI] [PubMed] [Google Scholar]
- 19.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Rhee JM, Heflin JA, Hamasaki T, Freedman B. Prevalence of physical signs in cervical myelopathy: A prospective, controlled study. Spine (Phila Pa 1976). 2009;34(9):890-895. doi: 10.1097/BRS.0b013e31819c944b. [DOI] [PubMed] [Google Scholar]
- 21.Cook C, Brown C, Isaacs R, Roman M, Davis S, Richardson W. Clustered clinical findings for diagnosis of cervical spine myelopathy. J Man Manip Ther. 2010;18(4):175-180. doi: 10.1179/106698110X12804993427045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaiyamongkol W, Laohawiriyakamol T, Tangtrakulwanich B, Tanutit P, Bintachitt P, Siribumrungwong K. The significance of the tromner sign in cervical spondylotic myelopathy patient. Clin Spine Surg. 2017;30(9):E1315-E1320. 10.1097/BSD.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 23.Chang CW, Chang KY, Lin SM. Quantification of the Tromner signs: A sensitive marker for cervical spondylotic myelopathy. Eur Spine J. 2011;20(6):923-927. doi: 10.1007/s00586-010-1681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrop JS, Naroji S, Maltenfort M, et al. Cervical myelopathy: A clinical and radiographic evaluation and correlation to cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2010;35(6):620-624. doi: 10.1097/BRS.0b013e3181b723af. [DOI] [PubMed] [Google Scholar]
- 25.Harrop JS, Naroji S, Maltenfort M, et al. A clinical correlation research of the Hoffmann sign and neurological imaging findings in cervical spinal cord compression. World Neurosurg. 2019;128:e782-e786. doi: 10.1016/j.wneu.2019.04.254. [DOI] [PubMed] [Google Scholar]
- 26.Glaser JA, Cure JK, Bailey KL, Morrow DL. Cervical spinal cord compression and the Hoffmann sign. Iowa Orthop J. 2001;21:49-52. [PMC free article] [PubMed] [Google Scholar]
- 27.Grijalva RA, Hsu FP, Wycliffe ND, et al. Hoffmann sign: Clinical correlation of neurological imaging findings in the cervical spine and brain. Spine (Phila Pa 1976). 2015;40(7):475-479. doi: 10.1097/BRS.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 28.Archer KR, Bydon M, Khan I, et al. Development and validation of cervical prediction models for patient-reported outcomes at 1 Year after cervical spine surgery for radiculopathy and myelopathy. Spine (Phila Pa 1976). 2020;45(22):1541-1552. doi: 10.1097/BRS.0000000000003610. [DOI] [PubMed] [Google Scholar]
- 29.Phillips DG. Upper limb involvement in cervical spondylosis. J Neurol Neurosurg Psychiatry. 1975;38(4):386-390. doi: 10.1136/jnnp.38.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acharya S, Srivastava A, Virmani S, Tandon R. Resolution of physical signs and recovery in severe cervical spondylotic myelopathy after cervical laminoplasty. Spine (Phila Pa 1976). 2010;35(21):E1083-E1087. doi: 10.1097/BRS.0b013e3181df1a8e. [DOI] [PubMed] [Google Scholar]
- 31.Alafifi T, Kern R, Fehlings M. Clinical and MRI predictors of outcome after surgical intervention for cervical spondylotic myelopathy. J Neuroimaging. 2007;17(4):315-322. doi: 10.1111/j.1552-6569.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 32.Chikuda H, Seichi A, Takeshita K, et al. Correlation between pyramidal signs and the severity of cervical myelopathy. Eur Spine J. 2010;19(10):1684-1689. doi: 10.1007/s00586-010-1364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiles BW, 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: Patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44(4):762-769. doi: 10.1097/00006123-199904000-00041. [DOI] [PubMed] [Google Scholar]
- 34.Findlay GF, Balain B, Trivedi JM, Jaffray DC. Does walking change the Romberg sign? Eur Spine J. 2009;18(10):1528-1531. 10.1007/s00586-009-1008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funaba M, Imajo Y, Suzuki H, et al. The associations between radiological and neurological findings of degenerative cervical myelopathy: Radiological analysis based on kinematic CT myelography and evoked potentials of the spinal cord. J Neurosurg Spine. 2021:1-12. doi: 10.3171/2020.11.SPINE201626. [DOI] [PubMed] [Google Scholar]
- 36.Houten JK, Noce LA. Clinical correlations of cervical myelopathy and the Hoffmann sign. J Neurosurg Spine. 2008;9(3):237-242. doi: 10.3171/SPI/2008/9/9/237. [DOI] [PubMed] [Google Scholar]
- 37.Kara B, Celik A, Karadereler S, et al. The role of DTI in early detection of cervical spondylotic myelopathy: A preliminary study with 3-T MRI. Neuroradiology. 2011;53(8):609-616. doi: 10.1007/s00234-011-0844-4. [DOI] [PubMed] [Google Scholar]
- 38.Di Lazzaro V, Restuccia D, Colosimo C, Tonali P. The contribution of magnetic stimulation of the motor cortex to the diagnosis of cervical spondylotic myelopathy. Correlation of central motor conduction to distal and proximal upper limb muscles with clinical and MRI findings. Electroencephalogr Clin Neurophysiol. 1992;85(5):311-320. doi: 10.1016/0168-5597(92)90107-m. [DOI] [PubMed] [Google Scholar]
- 39.Paholpak P, Jirarattanaphochai K, Sae-Jung S, Wittayapairoj K. Clinical correlation of cervical myelopathy and the hyperactive pectoralis reflex. J Spinal Disord Tech. 2013;26(8):E314-E318. doi: 10.1097/BSD.0b013e3182886edb. [DOI] [PubMed] [Google Scholar]
- 40.Pilato F, Calandrelli R, Distefano M, Tamburrelli FC. Multidimensional assessment of cervical spondylotic myelopathy patients. Usefulness of a comprehensive score system. Neurol Sci. 2021;42(4):1507-1514. doi: 10.1007/s10072-020-04691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Restuccia D, Valeriani M, Di Lazzaro V, Tonali P, Mauguiere F. Somatosensory evoked potentials after multisegmental upper limb stimulation in diagnosis of cervical spondylotic myelopathy. J Neurol Neurosurg Psychiatry. 1994;57(3):301-308. doi: 10.1136/jnnp.57.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson JC, Broaddus WC, Smith MM, Kubal WS. Hyperactive pectoralis reflex as an indicator of upper cervical spinal cord compression. Report of 15 cases. J Neurosurg. 1997;86(1):159-161. doi: 10.3171/jns.1997.86.1.0159. [DOI] [PubMed] [Google Scholar]
- 43.Veilleux M, Daube JR. The value of ulnar somatosensory evoked potentials (SEPs) in cervical myelopathy. Electroencephalogr Clin Neurophysiol. 1987;68(6):415-423. doi: 10.1016/0168-5597(87)90053-0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang YZ, Shen Y, Wang LF, Ding WY, Xu JX, He J. Magnetic resonance T2 image signal intensity ratio and clinical manifestation predict prognosis after surgical intervention for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2010;35(10):E396-E399. doi: 10.1097/BRS.0b013e3181c6dbc4. [DOI] [PubMed] [Google Scholar]
- 45.Wong TM, Leung HB, Wong WC. Correlation between magnetic resonance imaging and radiographic measurement of cervical spine in cervical myelopathic patients. J Orthop Surg. 2004;12(2):239-242. doi: 10.1177/230949900401200220. [DOI] [PubMed] [Google Scholar]
- 46.Chibbaro S, Benvenuti L, Carnesecchi S, et al. Anterior cervical corpectomy for cervical spondylotic myelopathy: Experience and surgical results in a series of 70 consecutive patients. J Clin Neurosci. 2006;13(2):233-238. doi: 10.1016/j.jocn.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Lo YL, Chan LL, Lim W, et al. Systematic correlation of transcranial magnetic stimulation and magnetic resonance imaging in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2004;29(10):1137-1145. doi: 10.1097/00007632-200405150-00017. [DOI] [PubMed] [Google Scholar]
- 48.Misra UK, Kalita J. Motor evoked potential is useful for monitoring the effect of collar therapy in cervical spondylotic myelopathy. J Neurol Sci. 1998;154(2):222-228. doi: 10.1016/s0022-510x(97)00275-x. [DOI] [PubMed] [Google Scholar]
- 49.Stetkarova I, Kofler M. Cutaneous silent periods in the assessment of mild cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2009;34(1):34-42. doi: 10.1097/BRS.0b013e31818f8be3. [DOI] [PubMed] [Google Scholar]
- 50.Lyu RK, Tang LM, Chen CJ, Chen CM, Chang HS, Wu YR. The use of evoked potentials for clinical correlation and surgical outcome in cervical spondylotic myelopathy with intramedullary high signal intensity on MRI. J Neurol Neurosurg Psychiatry. 2004;75(2):256-261. [PMC free article] [PubMed] [Google Scholar]
- 51.Chacko AG, Joseph M, Turel MK, Prabhu K, Daniel RT, Jacob KS. Multilevel oblique corpectomy for cervical spondylotic myelopathy preserves segmental motion. Eur Spine J. 2012;21(7):1360-1367. doi: 10.1007/s00586-011-2137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wazir NN, Kareem BA. New clinical sign of cervical myelopathy: Wazir hand myelopathy sign. Singapore Med J. 2011;52(1):47-49. [PubMed] [Google Scholar]
- 53.Aggarwal RA, Srivastava SK, Bhosale SK, Nemade PS. Prediction of surgical outcome in compressive cervical myelopathy: A novel clinicoradiological prognostic score. J Craniovertebr Junction Spine. 2016;7(2):82-86. doi: 10.4103/0974-8237.181828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed OEF, Galal A. Multiple level anterior cervical discectomy and fusion versus posterior laminectomy for the management of multilevel cervical spondylotic myelopathy: clinical and radiological outcome. Egyptian Journal of Neurology, Psychiatry and Neurosurgery. 2020;56(32). [Google Scholar]
- 55.Audat ZA, Fawareh MD, Radydeh AM, et al. Anterior versus posterior approach to treat cervical spondylotic myelopathy, clinical and radiological results with long period of follow-up, 6. Sage Open Med; 2018. pp. 2050312118766199. 10.1177/2050312118766199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burkhardt BW, Brielmaier M, Schwerdtfeger K, Sharif S, Oertel JM. Smith-Robinson procedure with and without Caspar plating as a treatment for cervical spondylotic myelopathy: A 26-year follow-up of 23 patients. Eur Spine J. 2017;26(4):1246-1253. doi: 10.1007/s00586-017-4988-8. [DOI] [PubMed] [Google Scholar]
- 57.Chacko AG, Turel MK, Sarkar S, Prabhu K, Daniel RT. Clinical and radiological outcomes in 153 patients undergoing oblique corpectomy for cervical spondylotic myelopathy. Br J Neurosurg. 2014;28(1):49-55. doi: 10.3109/02688697.2013.815326. [DOI] [PubMed] [Google Scholar]
- 58.Chatley A, Kumar R, Jain VK, Behari S, Sahu RN. Effect of spinal cord signal intensity changes on clinical outcome after surgery for cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(5):562-567. doi: 10.3171/2009.6.SPINE091. [DOI] [PubMed] [Google Scholar]
- 59.Chibbaro S, Mirone G, Makiese O, George B. Multilevel oblique corpectomy without fusion in managing cervical myelopathy: Long-term outcome and stability evaluation in 268 patients. J Neurosurg Spine. 2009;10(5):458-465. doi: 10.3171/2009.1.SPINE08186. [DOI] [PubMed] [Google Scholar]
- 60.Chiu AYY, Pang MYC. Assessment of psychometric properties of various balance assessment tools in persons with cervical spondylotic myelopathy. J Orthop Sports Phys Ther. 2017;47(9):673-682. doi: 10.2519/jospt.2017.7283. [DOI] [PubMed] [Google Scholar]
- 61.Du W, Wang L, Shen Y, Zhang Y, Ding W, Ren L. Long-term impacts of different posterior operations on curvature, neurological recovery and axial symptoms for multilevel cervical degenerative myelopathy. Eur Spine J. 2013;22(7):1594-1602. doi: 10.1007/s00586-013-2741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Du W, Zhang P, Shen Y, Zhang YZ, Ding WY, Ren LX. Enlarged laminectomy and lateral mass screw fixation for multilevel cervical degenerative myelopathy associated with kyphosis. Spine J. 2014;14(1):57-64. doi: 10.1016/j.spinee.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 63.El-Ghandour NMF, Soliman MAR, Ezzat AAM, Mohsen A, Zein-Elabedin M. The safety and efficacy of anterior versus posterior decompression surgery in degenerative cervical myelopathy: A prospective randomized trial. J Neurosurg Spine. 2020:1-9. doi: 10.3171/2020.2.SPINE191272. [DOI] [PubMed] [Google Scholar]
- 64.Gembruch O, Jabbarli R, Rashidi A, et al. Degenerative cervical myelopathy in higher-aged patients: How do they benefit from surgery? J Clin Med. 2019;9(1). doi: 10.3390/jcm9010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerling MC, Radcliff K, Isaacs R, et al. Two-Year results of the prospective spine treatment outcomes study: An analysis of complication rates, predictors of their development, and effect on patient derived outcomes at 2 Years for surgical management of cervical spondylotic myelopathy. World Neurosurg. 2017;106:247-253. doi: 10.1016/j.wneu.2017.06.147. [DOI] [PubMed] [Google Scholar]
- 66.Holly LT, Ellingson BM, Salamon N. Metabolic imaging using proton magnetic spectroscopy as a predictor of outcome after surgery for cervical spondylotic myelopathy. Clin Spine Surg. 2017;30(5):E615-E619. doi: 10.1097/BSD.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;10(3):194-200. doi: 10.3171/2008.12.SPINE08367. [DOI] [PubMed] [Google Scholar]
- 68.Hossam M SM, Elsame M. Median cervical corpectomy for cervical myelopathy associated with ossified posterior longitudinal ligament. Trends Med Res. 2013;8:1-15. [Google Scholar]
- 69.Hou X, Lu S, Wang B, Kong C, Hu H. Morphologic characteristics of the deep cervical paraspinal muscles in patients with single-level cervical spondylotic myelopathy. World Neurosurg. 2020;134:e166-e171. doi: 10.1016/j.wneu.2019.09.162. [DOI] [PubMed] [Google Scholar]
- 70.Imajo Y, Kato Y, Yonemura H, Kanchiku T, Suzuki H, Taguchi T. Relative vulnerability of various spinal tracts in C3-4 cervical spondylotic myelopathy: Multi-modal spinal cord evoked potentials. Spinal Cord. 2011;49(11):1128-1133. doi: 10.1038/sc.2011.68. [DOI] [PubMed] [Google Scholar]
- 71.Jain S SS, Kumar Joshi A, Pamecha C, Dave B, Patel P. Comparative study of anterior versus posterior decompression in elderly patients of cervical myelopathy with co-morbid conditions. Eur J Orthop Surg Traumatol. 2009;19:397-401. [Google Scholar]
- 72.Kim CR, Yoo JY, Lee SH, Lee DH, Rhim SC. Gait analysis for evaluating the relationship between increased signal intensity on t2-weighted magnetic resonance imaging and gait function in cervical spondylotic myelopathy. Arch Phys Med Rehabil. 2010;91(10):1587-1592. doi: 10.1016/j.apmr.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 73.Kim YJ, Oh SH, Yi HJ, Kim YS, Ko Y, Oh SJ. Myelopathy caused by soft cervical disc herniation: Surgical results and prognostic factors. J Korean Neurosurg Soc. 2007;42(6):441-445. doi: 10.3340/jkns.2007.42.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kong W, Xin Z, Du Q, Cao G, Liao W. Anterior percutaneous full-endoscopic transcorporeal decompression of the spinal cord for single-segment cervical spondylotic myelopathy: The technical interpretation and 2 years of clinical follow-up. J Orthop Surg Res. 2019;14(1):461. doi: 10.1186/s13018-019-1474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409. doi: 10.1016/j.jocn.2008.07.070. [DOI] [PubMed] [Google Scholar]
- 76.Lo YL, Tan YE, Dan YF, et al. Cutaneous silent periods in the evaluation of cord compression in cervical spondylosis. J Neurol. 2007;254(1):14-19. doi: 10.1007/s00415-007-0142-6. [DOI] [PubMed] [Google Scholar]
- 77.Lu K, Gao X, Tong T, Miao D, Ding W, Shen Y. Clinical predictors of surgical outcomes and imaging features in single segmental cervical spondylotic myelopathy with lower cervical instability. Med Sci Monit. 2017;23:3697-3705. doi: 10.12659/msm.906046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moussellard HP, Meyer A, Biot D, Khiami F, Sariali E. Early neurological recovery course after surgical treatment of cervical spondylotic myelopathy: A prospective study with 2-year follow-up using three different functional assessment tests. Eur Spine J. 2014;23(7):1508-1514. doi: 10.1007/s00586-014-3315-x. [DOI] [PubMed] [Google Scholar]
- 79.Raslan F, Koehler S, Berg F, et al. Vertebral body replacement with PEEK-cages after anterior corpectomy in multilevel cervical spinal stenosis: A clinical and radiological evaluation. Arch Orthop Trauma Surg. 2014;134(5):611-618. 10.1007/s00402-014-1972-1. [DOI] [PubMed] [Google Scholar]
- 80.Revanappa KK, Moorthy RK, Alexander M, Rajshekhar V. Recovery of sympathetic skin response after central corpectomy in patients with moderate and severe cervical spondylotic myelopathy. Br J Neurosurg. 2017;31(2):199-204. doi: 10.1080/02688697.2016.1206178. [DOI] [PubMed] [Google Scholar]
- 81.Scholler K, Siller S, Brem C, Lutz J, Zausinger S. Diffusion tensor imaging for surgical planning in patients with cervical spondylotic myelopathy. J Neurol Surg Cent Eur Neurosurg. 2020;81(1):1-9. doi: 10.1055/s-0039-1691822. [DOI] [PubMed] [Google Scholar]
- 82.Sinha S, Jagetia A. Bilateral open-door expansive laminoplasty using unilateral posterior midline approach with preservation of posterior supporting elements for management of cervical myelopathy and radiculomyelopathy--analysis of clinical and radiological outcome and surgical technique. Acta Neurochir. 2011;153(5):975-984. doi: 10.1007/s00701-010-0872-6. [DOI] [PubMed] [Google Scholar]
- 83.Sugawara T, Hirano Y, Higashiyama N, Mizoi K. Limaprost alfadex improves myelopathy symptoms in patients with cervical spinal canal stenosis. Spine (Phila Pa 1976). 2009;34(6):551-555. doi: 10.1097/BRS.0b013e31819a84ec. [DOI] [PubMed] [Google Scholar]
- 84.Turel MK, Sarkar S, Prabhu K, Daniel RT, Jacob KS, Chacko AG. Reduction in range of cervical motion on serial long-term follow-up in patients undergoing oblique corpectomy for cervical spondylotic myelopathy. Eur Spine J. 2013;22(7):1509-1516. doi: 10.1007/s00586-013-2724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, Liu CY, Tian JW, Tian X, Dong SH, Zhao QH. Combined single-level subtotal corpectomy and decompression for cervical spondylotic myelopathy treatment. ANZ J Surg. 2012;82(5):342-347. doi: 10.1111/j.1445-2197.2011.05996.x. [DOI] [PubMed] [Google Scholar]
- 86.Wei L, Cao P, Xu C, et al. The relationship between preoperative factors and the presence of intramedullary increased signal intensity on T2-weighted magnetic resonance imaging in patients with cervical spondylotic myelopathy. Clin Neurol Neurosurg. 2019;178:1-6. doi: 10.1016/j.clineuro.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 87.Williams KE, Paul R, Dewan Y. Functional outcome of corpectomy in cervical spondylotic myelopathy. Indian J Orthop. 2009;43(2):205-209. doi: 10.4103/0019-5413.50855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vitzthum HE, Dalitz K.. Analysis of five specific scores for cervical spondylogenic myelopathy. Eur Spine J. 2007;16(12):2096-2103. doi: 10.1007/s00586-007-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thakar S, Christopher S, Rajshekhar V. Quality of life assessment after central corpectomy for cervical spondylotic myelopathy: Comparative evaluation of the 36-item short form health survey and the world health organization quality of life-bref. J Neurosurg Spine. 2009;11(4):402-412. doi: 10.3171/2009.4.SPINE08749. [DOI] [PubMed] [Google Scholar]
- 90.!!! INVALID CITATION !!! .
- 91.Malanga GA, Landes P, Nadler SF. Provocative tests in cervical spine examination: Historical basis and scientific analyses. Pain Physician. 2003;6(2):199-205. [PubMed] [Google Scholar]
- 92.Dafkin C, Green A, Kerr S, Veliotes D, Olivier B, McKinon W. The interrater reliability of subjective assessments of the Babinski reflex. J Mot Behav. 2016;48(2):116-121. doi: 10.1080/00222895.2015.1047486. [DOI] [PubMed] [Google Scholar]
- 93.Miller TM, Johnston SC. Should the Babinski sign be part of the routine neurologic examination? Neurology. 2005;65(8):1165-1168. doi: 10.1212/01.wnl.0000180608.76190.10. [DOI] [PubMed] [Google Scholar]
- 94.Matsumoto M, Fujimura Y, Toyama Y. Usefulness and reliability of neurological signs for level diagnosis in cervical myelopathy caused by soft disc herniation. J Spinal Disord. 1996;9(4):317-321. [PubMed] [Google Scholar]
- 95.Kuhtz-Buschbeck JP, Johnk K, Mader S, Stolze H, Mehdorn M. Analysis of gait in cervical myelopathy. Gait Posture. 1999;9(3):184-189. doi: 10.1016/s0966-6362(99)00015-6. [DOI] [PubMed] [Google Scholar]
- 96.Haddas R, Patel S, Arakal R, Boah A, Belanger T, Ju KL. Spine and lower extremity kinematics during gait in patients with cervical spondylotic myelopathy. Spine J. 2018;18(9):1645-1652. doi: 10.1016/j.spinee.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 97.Lee JH, Lee SH, Seo IS. The characteristics of gait disturbance and its relationship with posterior tibial somatosensory evoked potentials in patients with cervical myelopathy. Spine (Phila Pa 1976). 2011;36(8):E524-E530. doi: 10.1097/BRS.0b013e3181f412d9. [DOI] [PubMed] [Google Scholar]
- 98.Malone A, Meldrum D, Bolger C. Gait impairment in cervical spondylotic myelopathy: Comparison with age- and gender-matched healthy controls. Eur Spine J. 2012;21(12):2456-2466. doi: 10.1007/s00586-012-2433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suzuki E, Nakamura H, Konishi S, Yamano Y. Analysis of the spastic gait caused by cervical compression myelopathy. J Spinal Disord Tech. 2002;15(6):519-522. doi: 10.1097/00024720-200212000-00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The Value of Clinical Signs in the Diagnosis of Degenerative Cervical Myelopathy: Results of a Systematic Review by Zhilin Jiang, Benjamin Davies, Carl Zipser, Konstantinos Margetis, Allan Martin, Stavros Matsoukas, Freschta Zipser-Mohammadzada, Najmeh Kheram, Andrea Boraschi, Elina Zakin, Oke Righteous Obadaseraye, Michael G. Fehlings, Jamie Wilson, Ratko Yurac, Chad E Cook, Jamie Milligan, Julia Tabrah, Shirley Widdop, Lianne Wood, Elizabeth A. Roberts, Tanzil Rujeedawa, Lindsay Tetreault on behalf of the AO Spine RECODE-DCM Diagnostic Criteria Incubator in Global Spine Journal