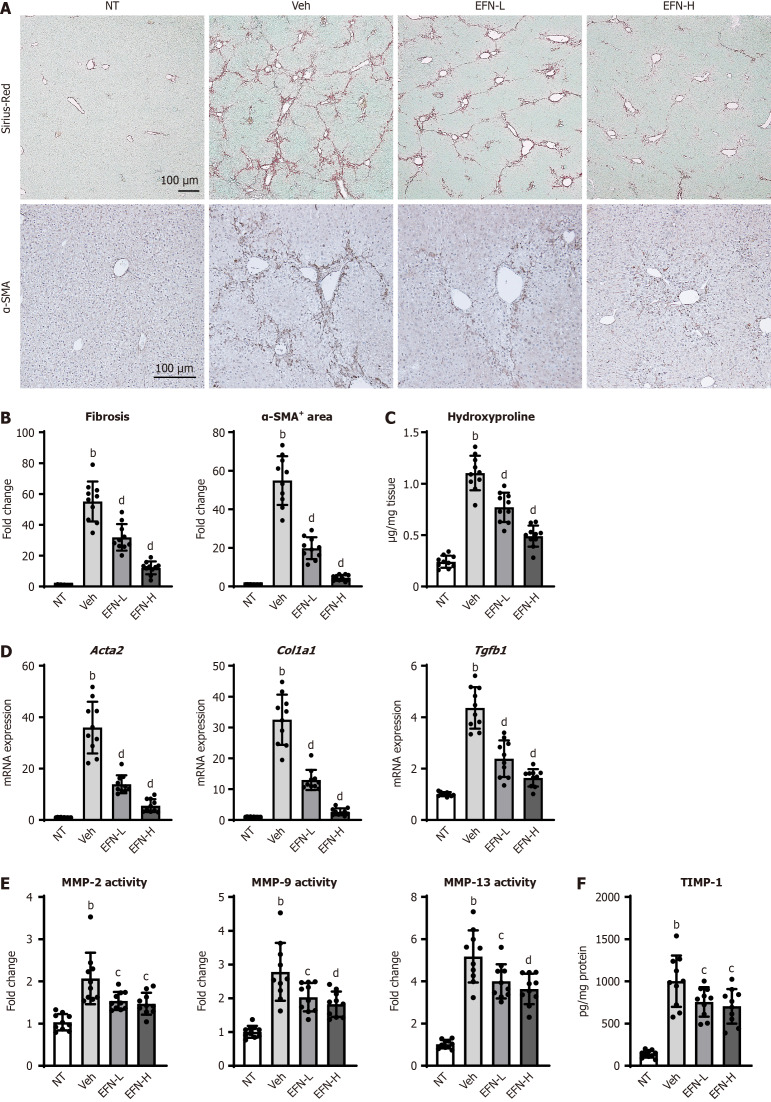
Figure 3.
Elafibranor on hepatic fibrosis development in the alcohol-associated liver disease mice. A: Representative microphotographs of sirius-red and α-smooth muscle actin (SMA) staining of the livers in the experimental mice; B: Quantification of sirius-red stained fibrotic area and α-SMA-positive area in high-power field (n = 10); C: Hepatic concentration of hydroxyproline (n = 10); D: Hepatic mRNA level of profibrotic markers (n = 10); E: Hepatic activity of matrix metalloproteinases (MMP)-2, MMP-9, and MMP-13 (n = 10); F: Hepatic level of tissue inhibitor of metalloproteinase 1 (n = 10). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction (D). Quantitative values are indicated as fold changes to the values of non-therapeutic group (B, D and E). Data are the mean ± SD. bP < 0.01 vs non-therapeutic group; cP < 0.05 vs vehicle-treated alcohol-associated liver disease group; dP < 0.01 vs vehicle-treated alcohol-associated liver disease group, significant difference between groups by Student’s t-test. NT: Non-therapeutic group; Veh: Vehicle-treated alcohol-associated liver disease group; EFN-L: Elafiblanor (3 mg/kg/day)-treated alcohol-associated liver disease group; EFN-H: Elafibranor (10 mg/kg/day)-treated alcohol-associated liver disease group; α-SMA: α-smooth muscle actin; MMP: Matrix metalloproteinases; TIMP1: Tissue inhibitor of metalloproteinase 1.