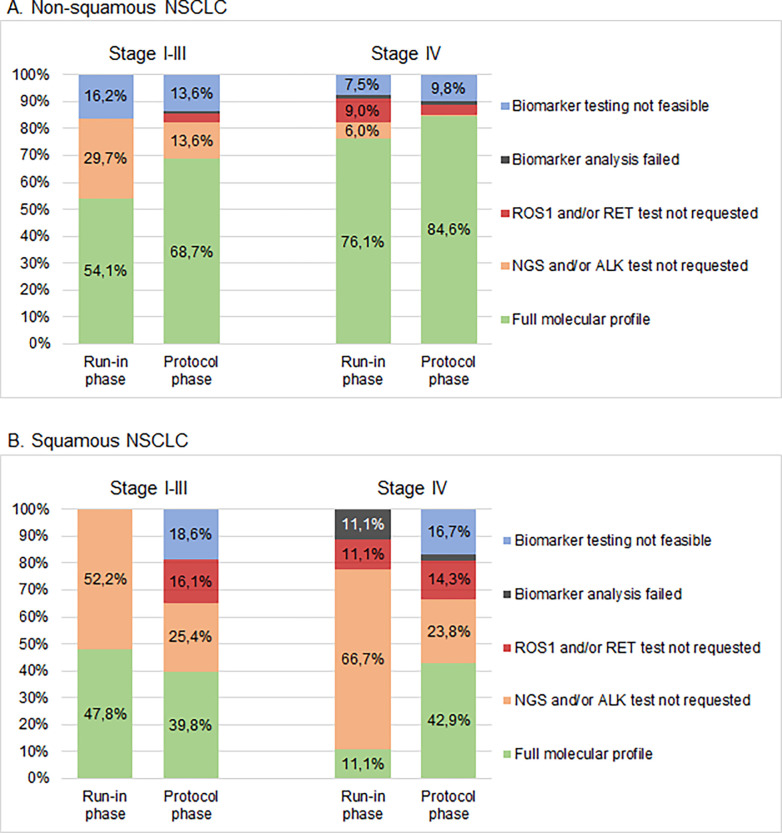
Fig 3. Comprehensive molecular profiling rate.
The proportion of patients in whom comprehensive molecular profiling was feasible in: (A) non-squamous cell carcinoma. Stage I-III: run-in phase n = 37, protocol phase n = 294. Stage IV: run-in phase n = 67, protocol phase n = 286. (B) squamous cell carcinoma. Stage I-III: run-in phase n = 23, protocol phase n = 119. Stage IV: run-in phase n = 9, protocol phase n = 42. All absolute numbers and percentages are shown in S3 Table.