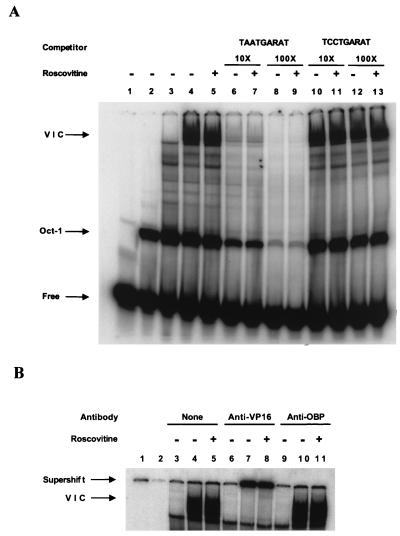
FIG. 2.
(A) Roscovitine does not affect induction of virion-induced TAATGARAT DNA binding activity. Three microliters of nuclear extract (12 μg of protein) was incubated in 12 μl of binding buffer [10 mM HEPES (pH 8.0), 1 mM EDTA, 5 mM dithiothreitol, 0.1% NP-40, 0.5% Ficoll, 50 ng of salmon sperm DNA/ml, 1.5 μg of poly(dIdC)/ml] for 5 min at 20°C. In addition, the binding reaction mixture was supplemented with histidine-tagged Oct-1 POU domain protein expressed in E. coli and purified by nickel affinity chromatography. After 5 min of incubation, 0.5 ng of a 32P-end-labeled (∼5 × 105 cpm) 29-bp oligonucleotide probe (see text for complete sequence) containing the wild-type VP16 response element from the ICP0 promoter (TAATGARAT) or a probe containing a mutant VP16 response element (TCCTGARAT) was added to the reaction mixture, and the mixture was incubated for an additional 30 min at 20°C. The protein-DNA complexes were separated by native gel electrophoresis on a 5% polyacrylamide gel in TBE buffer and visualized by PhosphorImager analysis. Lane 1, free probe; lane 2, Oct-POU domain (Oct-1); lane 3, mock-infected nuclear extract; lanes 4 to 13, KOS-infected nuclear extract in the presence (+) or absence (−) of 100 μM Roscovitine. The VIC is indicated. (B) VP16 antibody supershifts the VIC. Binding reactions were performed as described for panel A. Following the 30-min incubation period, 1 μl of binding buffer (lanes 3 to 5), polyclonal rabbit antibody directed against a Gal4-VP16 fusion protein (32) (anti-VP16) (lanes 6 to 8), or antibody directed against the HSV-1 origin binding protein (anti-OBP) (lanes 9 to 11) was added to the reaction mixtures. The binding reaction mixtures were incubated for 5 min prior to gel electrophoresis. Lane 1, free probe (not shown in figure); lane 2, Oct-1 (not shown in figure); lanes 3, 6, and 9, mock-infected extracts; lanes 4, 5, 7, 8, 10, and 11, KOS-infected nuclear extract in the presence (+) or absence (−) of 100 μM Roscovitine.