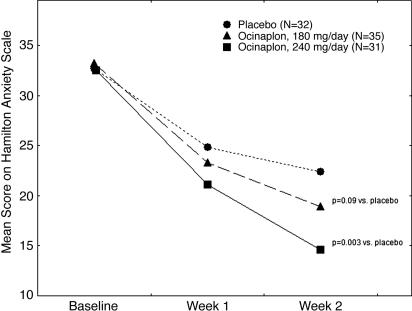
Fig. 4.
Anxiolytic effects of ocinaplon in GAD. Eligible patients with a diagnosis (HAM-A score of ≥20 at the end of the 1-week placebo run-in period) of GAD were randomized into three groups receiving placebo, 180 mg of ocinaplon (60 mg three times daily), or 240 mg of ocinaplon (120 mg twice daily) for 14 days. For each group and each time point, the depicted values represent the mean total score on the HAM-A scale of evaluable patients completing the study with no protocol deviation. The overall effect of ocinaplon on HAM-A scores was significantly different (P = 0.007) compared with placebo. Filled circles, placebo; open circles, ocinaplon, 180 mg/day; filled squares, ocinaplon 240 mg/day; a: t = 1.7, df = 66, P = 0.09 for Ocinaplon, 180 mg/day vs. placebo; b, t = 3.1, df = 62, P = 0.003 for Ocinaplon 240 mg/day vs. placebo. These t values represent the dose × time interaction for each individual dose of ocinaplon.