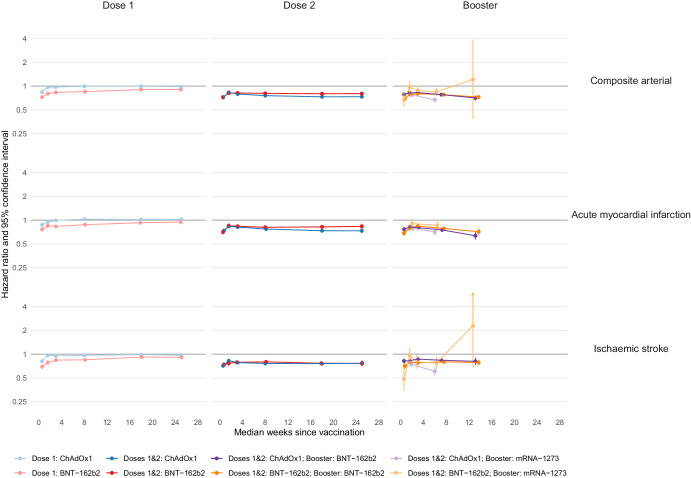
Fig. 1. Adjusted hazard ratios (aHRs) and 95% confidence intervals (95% CIs) for arterial thromboses following COVID-19 vaccination, by dose and brand.
Vertical lines depict 95% CIs; these are not visible when they are very narrow. There were no AMI events during weeks 13–26 after mRNA-1273 booster vaccination, so follow-up is grouped as 1–4 and 5–26 weeks post-vaccination. The number of people eligible for first, second, and booster dose analyses were 45,673,965; 37,249,850 and 35,853,120, respectively. The number of people who received a first dose of ChAdOx1, BNT-162b2 and mRNA-1273 were 19,317,985, 16,846,995, and 1,084,865, respectively; a second dose of ChAdOx1, BNT-162b2 and mRNA-1273 were 18,920,225, 15,961,330, and 971,565, respectively; a booster dose of BNT-162b2 and mRNA-1273 following a primary course of ChAdOx1 were 11,964,635and 4,153,760 respectively; a booster dose of BNT-162b2 and mRNA-1273 following a primary course of BNT-162b2 were 9,821,835 and 1,914,925, respectively. The numerical values of hazard ratios and 95% CIs are displayed in Supplementary Tables 8, 9, 11, 12, 14, 15, 17 and 18.